��Ŀ����
�ڻ�ѧʵ�鼼�ܿ������ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д������������Ȼ��ƵĻ����Һ(��������������)��Ϊ������Ⱦ�������������÷�Һ����ѧ��ȤС����������ʵ�飺ȡ��ҺͰ�ϲ���Һ��11.88kg�������м���������������Ϊ21.2����̼������Һ��������ҺpH������̼������Һ��������ϵ����ͼ��ʾ
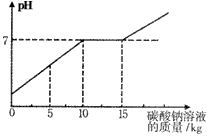
(1)ͨ����ͼ��֪����̼������Һ�����ӵ�____________kgʱ����Һǡ�ô�����(�������Ȼ��ƵĻ����Һ��ȫת�����Ȼ�����Һ)��
(2)��ʱ������Һ�ܷ����ڸ�У������ȤС���С��ѡ��(ѡ��ҺҪ���Ȼ��Ƶ���������������10��һ20��֮��)?��ͨ������ش�
(2)��ʱ������Һ�ܷ����ڸ�У������ȤС���С��ѡ��(ѡ��ҺҪ���Ȼ��Ƶ���������������10��һ20��֮��)?��ͨ������ش�
(1)15kg
(2)�⣺��Na2CO3��HCl��Ӧ����NaCl��CO2�������ֱ�Ϊx1��y1
Na2CO3+2HCl=2NaCl+CO2��ʮH2O
106 117 44
10kg��21.2�� x1 y1
106/117=(10kg��21.2��)/ x1 x1=2.34kg
106/44=(10kg��21.2��)/y1 y1=0.88kg
��Na2CO3��CaCl2��Ӧ����NaCl��CaCO3�������ֱ�Ϊx2��y2
Na2CO3+CaCl2=CaCO3��+2NaCl
106 100 117
5kg��21.2�� y2 x2
106/117=(5kg��21.2��)/x2 x2=1.17kg
106/100=(5kg��21.2��)/y2 y2=1kg
ǡ�ô�����ʱ��Һ��������������=(2.34kg+1.17kg)/(11.88kg+15kg-0.88kg-1kg)��100%=14.04��
�𣺿�����Ϊ������ȤС���ѡ��Һ��
(2)�⣺��Na2CO3��HCl��Ӧ����NaCl��CO2�������ֱ�Ϊx1��y1
Na2CO3+2HCl=2NaCl+CO2��ʮH2O
106 117 44
10kg��21.2�� x1 y1
106/117=(10kg��21.2��)/ x1 x1=2.34kg
106/44=(10kg��21.2��)/y1 y1=0.88kg
��Na2CO3��CaCl2��Ӧ����NaCl��CaCO3�������ֱ�Ϊx2��y2
Na2CO3+CaCl2=CaCO3��+2NaCl
106 100 117
5kg��21.2�� y2 x2
106/117=(5kg��21.2��)/x2 x2=1.17kg
106/100=(5kg��21.2��)/y2 y2=1kg
ǡ�ô�����ʱ��Һ��������������=(2.34kg+1.17kg)/(11.88kg+15kg-0.88kg-1kg)��100%=14.04��
�𣺿�����Ϊ������ȤС���ѡ��Һ��

��ϰ��ϵ�д�
�����Ŀ
 �ڻ�ѧʵ�鼼�ܿ������ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ����ѧ��ȤС����������ʵ�飺
�ڻ�ѧʵ�鼼�ܿ������ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ����ѧ��ȤС����������ʵ�飺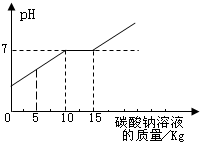 �ڻ�ѧʵ�鼼�ܿ������ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ����ѧ��ȤС����������ʵ�飺
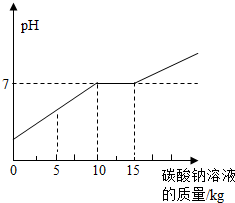
�ڻ�ѧʵ�鼼�ܿ������ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ����ѧ��ȤС����������ʵ�飺 ��2012?��ͷ������ģ���ڻ�ѧʵ�鼼�ܿ������ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ����ѧ��ȤС����������ʵ�飺ȡ��Һ10kg�������м���������������Ϊ21.2%��̼������Һ��������ҺpH������̼������Һ��������ϵ����ͼ��ʾ����֪��Na2CO3+CaCl2=CaCO3��+2NaCl����
��2012?��ͷ������ģ���ڻ�ѧʵ�鼼�ܿ������ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ����ѧ��ȤС����������ʵ�飺ȡ��Һ10kg�������м���������������Ϊ21.2%��̼������Һ��������ҺpH������̼������Һ��������ϵ����ͼ��ʾ����֪��Na2CO3+CaCl2=CaCO3��+2NaCl���� ��2013?����ģ�⣩�ڻ�ѧʵ�鼼�ܿ������ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ����ѧ��ȤС����������ʵ�飺ȡ��Һ10kg�������м���������������Ϊ21.2%��̼������Һ��������ҺpH������̼������Һ��������ϵ��ͼ��ʾ����֪��Na2CO3+CaCl2=CaCO3��+2NaCl����
��2013?����ģ�⣩�ڻ�ѧʵ�鼼�ܿ������ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ����ѧ��ȤС����������ʵ�飺ȡ��Һ10kg�������м���������������Ϊ21.2%��̼������Һ��������ҺpH������̼������Һ��������ϵ��ͼ��ʾ����֪��Na2CO3+CaCl2=CaCO3��+2NaCl����