��Ŀ����
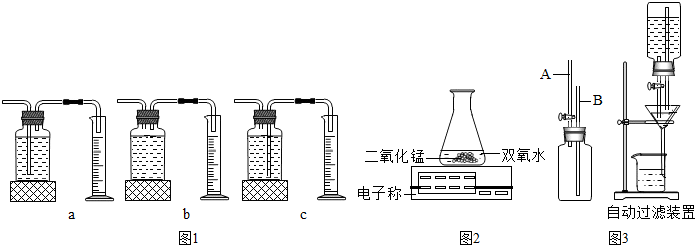
 ijͬѧ������ø��������ȡ���ռ�������װ����ͼ��
ijͬѧ������ø��������ȡ���ռ�������װ����ͼ����1��д��ͼ�б�����ŵ��������ƣ�
��
�ƾ���
�ƾ���
������ƿ
����ƿ
������̨
����̨
����2��ָ������ȡװ�ã���ߣ��е��������Դ���
��
�Թܿ�û����������б
�Թܿ�û����������б
�����Թܿ�û��һ����
�Թܿ�û��һ����
���Ӿƾ�����δ��ȼ�Ƕȿ������ռ�װ���л���������������
��
����ƿ��ˮû����
����ƿ��ˮû����
����û��ʼ���ȾͰѵ����쵽����ƿ��
û��ʼ���ȾͰѵ����쵽����ƿ��
����3����ȡ�����IJ��������У�A�����Թ���װ�������� B�������Թ� C�����װ�������� D�������е��ܵ���Ƥ�������Թܣ����̶�������̨�� E������������ʢ��ˮ�ļ���ƿ�� F���Թܿڷ�һ��������ȷ����˳����
CAFDBE
CAFDBE
����4����Ӧ�����ֱ���ʽ��
�������
�����+��������+����
| ���� |
�������
�����+��������+����
��| ���� |
��5��ʵ����Ϻ�Ӧ��
�ѵ����Ƴ�ˮ��
�ѵ����Ƴ�ˮ��
����Ϩ��ƾ���
Ϩ��ƾ���
���Է�ֹˮ�������Թܣ�ʹ�Թ�ը��
ˮ�������Թܣ�ʹ�Թ�ը��
����������1������ʵ���ҳ�����������ʶ������⣻
��2�������ø��������ȡ�����IJ���ע�����
��3��������ȡ������ʵ�鲽�裺��װ��������Ϩ�������⣻
��4�����ݸ��������ȡ�����ķ�Ӧ���ֱ���ʽ�ش�
��5�������ø��������ȡ�����IJ���ע�������Ӧ���ʱΪ�˷�ֹ�¶Ƚ��͵���ˮ�������Թ������Թ�ը�ѣ�����Ҫ�ȳ�������Ϩ�ƾ��ƣ�
��2�������ø��������ȡ�����IJ���ע�����
��3��������ȡ������ʵ�鲽�裺��װ��������Ϩ�������⣻
��4�����ݸ��������ȡ�����ķ�Ӧ���ֱ���ʽ�ش�
��5�������ø��������ȡ�����IJ���ע�������Ӧ���ʱΪ�˷�ֹ�¶Ƚ��͵���ˮ�������Թ������Թ�ը�ѣ�����Ҫ�ȳ�������Ϩ�ƾ��ƣ�
����⣺��1������ʵ���ҳ�����������ʶ������⣬��ˢ��Ǿƾ��ƣ����Ǽ���ƿ����������̨��
��2���ø��������ȡ����ʱ��Ӧע���Թܿ���������б���Է�����ˮ�������Թܵײ�ը���Թܣ��Թܿڷ�һ�������Է�������ط�ĩ���뵼�ܣ����ռ�ǰ����ƿ��ˮҪ�����������ܿ����������������ȵIJ���ʱ���ٰѵ����쵽����ƿ�У�
��3��ʵ�����ø��������ȡ������ʵ�鲽���Ǽ��װ�õ������ԣ�Ȼ�����Թ���װ�������أ��Թܿڷ�һ������Ȼ���е��ܵ���Ƥ�������Թܣ����̶�������̨�ϣ��̶����þƾ��Ƽ��ȣ�Ȼ������ˮ���ռ����壬������ʱ�Ƚ������Ƴ�ˮ����Ϩ��ƾ��ƣ�
��4��������طֽ���ȡ�����ķ�Ӧ�����ֱ���ʽ�Ǹ������
�����+��������+������
��5���ø��������ȡ����ʱ����Ӧ��Ϻ�Ϊ�˷�ֹ�¶Ƚ��͵���ˮ�������Թ������Թ�ը�ѣ�����Ҫ�ȳ�������Ϩ�ƾ��ƣ�
��1���ƾ��ƣ�����ƿ������̨��
��2���Թܿ�û����������б���Թܿ�û��һ����������ƿ��ˮû������û��ʼ���ȾͰѵ����쵽����ƿ�У�
��3��CAFDBE��
��4���������
�����+��������+������
��5���ѵ����Ƴ�ˮ�棻Ϩ��ƾ��ƣ�ˮ�������Թܣ�ʹ�Թ�ը�ѣ�
��2���ø��������ȡ����ʱ��Ӧע���Թܿ���������б���Է�����ˮ�������Թܵײ�ը���Թܣ��Թܿڷ�һ�������Է�������ط�ĩ���뵼�ܣ����ռ�ǰ����ƿ��ˮҪ�����������ܿ����������������ȵIJ���ʱ���ٰѵ����쵽����ƿ�У�
��3��ʵ�����ø��������ȡ������ʵ�鲽���Ǽ��װ�õ������ԣ�Ȼ�����Թ���װ�������أ��Թܿڷ�һ������Ȼ���е��ܵ���Ƥ�������Թܣ����̶�������̨�ϣ��̶����þƾ��Ƽ��ȣ�Ȼ������ˮ���ռ����壬������ʱ�Ƚ������Ƴ�ˮ����Ϩ��ƾ��ƣ�
��4��������طֽ���ȡ�����ķ�Ӧ�����ֱ���ʽ�Ǹ������
| ���� |
��5���ø��������ȡ����ʱ����Ӧ��Ϻ�Ϊ�˷�ֹ�¶Ƚ��͵���ˮ�������Թ������Թ�ը�ѣ�����Ҫ�ȳ�������Ϩ�ƾ��ƣ�
��1���ƾ��ƣ�����ƿ������̨��
��2���Թܿ�û����������б���Թܿ�û��һ����������ƿ��ˮû������û��ʼ���ȾͰѵ����쵽����ƿ�У�
��3��CAFDBE��
��4���������
| ���� |
��5���ѵ����Ƴ�ˮ�棻Ϩ��ƾ��ƣ�ˮ�������Թܣ�ʹ�Թ�ը�ѣ�
��������������ʵ��������ȡ������ԭ���Ͳ������裬�ر���ʵ�����ʱһ��Ҫ�ȳ����ܣ���Ϩ��ƾ��ƣ��Է�ˮ���������Թ�ը�ѣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
ij��ȤС��ͬѧ��ʵ������ȡ������������������̽��ʵ�飮
��1����֪2KClO3 2KCl+3O2����CuOҲ��������طֽ�Ĵ�������ͬѧΪ̽�����������������طֽ����ʵ�Ӱ�죬��������¶Ա����飺
2KCl+3O2����CuOҲ��������طֽ�Ĵ�������ͬѧΪ̽�����������������طֽ����ʵ�Ӱ�죬��������¶Ա����飺
I����xgKClO3��1.0gMnO2���Ȼ�ϼ��� II����3.0gKClO3��1.0gCuO���Ȼ�ϼ���
����ͬ�¶��£��Ƚ�����ʵ�����O2�Ŀ�����
��I��x��ֵΪ ����II�е�CuO��ϡ���ᷴӦ�ķ���ʽΪ ��
��2����ͬѧ̽����Ӱ��˫��ˮ�ֽ��ٶȵ�ij�����أ�ʵ�����ݼ�¼�����
�����ݴ��������ۡ�
�ٱ�ʵ���У�����O2�����װ���� �����ţ���

��ʵ����ۣ�����ͬ�����£� ��˫��ˮ�ֽ�ÿ죮
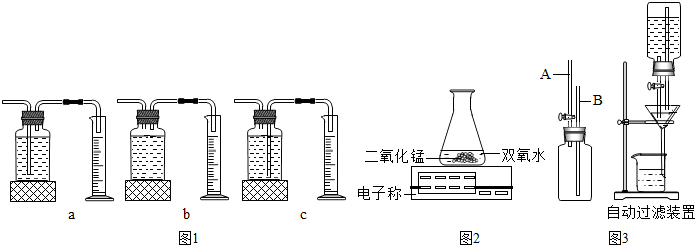
�۱�����ͼ2װ�ý���ʵ�飬ͨ���Ƚ� Ҳ�ܴﵽʵ��Ŀ�ģ�
��3��������غͶ������̵Ļ������ȡO2����ȫ��Ӧ��Ĺ���������������IJ�ʵ��������ɻ��յõ��ϴ����Ķ������̣�������ֻ�ж�����������ˮ������ȷ�������Ⱥ�˳���� ����дѡ����ţ���
a����� b���ܽ� c������ d��ϴ��
��4��ijͬѧ������ѧ֪ʶ�����һ���Զ���Һ����װ�ã�����ͨ����װ�õ�©���ϰ�װһ���Զ���Һװ�ã�ͼ3����ʹ��ʱ����Һ��������������ֽϿ죬���������������չˣ��Զ���Һװ����A��Ϊ��Һ�������ܡ���B��Ϊ����������ܡ��� A���Ͽڸ߳�B�ܿ�Լ1cm���Իش����⣺A���Ͽڸ߳�B���Ͽڵ������� ��
��1����֪2KClO3
 2KCl+3O2����CuOҲ��������طֽ�Ĵ�������ͬѧΪ̽�����������������طֽ����ʵ�Ӱ�죬��������¶Ա����飺
2KCl+3O2����CuOҲ��������طֽ�Ĵ�������ͬѧΪ̽�����������������طֽ����ʵ�Ӱ�죬��������¶Ա����飺I����xgKClO3��1.0gMnO2���Ȼ�ϼ��� II����3.0gKClO3��1.0gCuO���Ȼ�ϼ���
����ͬ�¶��£��Ƚ�����ʵ�����O2�Ŀ�����
��I��x��ֵΪ ����II�е�CuO��ϡ���ᷴӦ�ķ���ʽΪ ��
��2����ͬѧ̽����Ӱ��˫��ˮ�ֽ��ٶȵ�ij�����أ�ʵ�����ݼ�¼�����
| ˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2������ | ��ͬʱ���ڲ���O2����� | |
| I | 50.0g | 1% | 0.1g | 9mL |
| II | 50.0g | 2% | 0.1g | 16mL |
| III | 50.0g | 4% | 0.1g | 31mL |
�ٱ�ʵ���У�����O2�����װ���� �����ţ���

��ʵ����ۣ�����ͬ�����£� ��˫��ˮ�ֽ�ÿ죮
�۱�����ͼ2װ�ý���ʵ�飬ͨ���Ƚ� Ҳ�ܴﵽʵ��Ŀ�ģ�
��3��������غͶ������̵Ļ������ȡO2����ȫ��Ӧ��Ĺ���������������IJ�ʵ��������ɻ��յõ��ϴ����Ķ������̣�������ֻ�ж�����������ˮ������ȷ�������Ⱥ�˳���� ����дѡ����ţ���
a����� b���ܽ� c������ d��ϴ��
��4��ijͬѧ������ѧ֪ʶ�����һ���Զ���Һ����װ�ã�����ͨ����װ�õ�©���ϰ�װһ���Զ���Һװ�ã�ͼ3����ʹ��ʱ����Һ��������������ֽϿ죬���������������չˣ��Զ���Һװ����A��Ϊ��Һ�������ܡ���B��Ϊ����������ܡ��� A���Ͽڸ߳�B�ܿ�Լ1cm���Իش����⣺A���Ͽڸ߳�B���Ͽڵ������� ��