��Ŀ����
����Ŀ����2016������ʡ��20��ʱ������ͬ������NaCl����ֱ����100gˮ�У�����ܽ��������Һ���������NaCl���������Ķ�Ӧ��ϵ���±���

��ش��������⣬
��1��A�����ҺΪ ������͡������͡�����Һ��
��2��C��X��ֵΪ ��
��3����ͼΪNaCl���ܽ������ͼ��ͼ��a���������ֵΪ ��
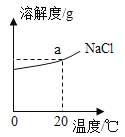
Ca(OH)2���ܽ�����¶ȱ仯��������NaCl�෴��������ʱ����ʯ��ˮ�м���������ʯ�ң����ָ������£���ʱ��Һ�����ʵ����� �����������������=����ԭ��Һ�����ʵ�������
���𰸡���1�������� ��2��120 ��3��36g������
����������1�����ݱ������ݿ�֪A��ʱ����100gˮ�л����Լ����ܽ����ʣ����A�����ҺΪ��������Һ��
��2��C�����ݼ����NaCl��������15��30g֮�䣬������20g������ҺΪ120g��
��3�����ݱ������ݿ�֪��20��ʱ100gˮ�������ܽ�36gNaCl������ͼ��a���������ֵΪ36g��Ca(OH)2���ܽ�����¶ȱ仯��������NaCl�෴��������ʱ����ʯ��ˮ�м���������ʯ�ң��ᷢ����Ӧ��CaO+H2O=Ca(OH)2�����ָ������£������ܼ����٣����Դ�ʱ��Һ�����ʵ�������ԭ��Һ�����ʵ�������