��Ŀ����
(4��).��һ����ɫ��ĩ�����ܺ���CuSO4��NaCl��AgCl��Na2SO4��Na2CO3�е�һ�ֻ��֣��ֽ������µ�ʵ�飺��1������ɫ��ĩ����������ˮ�У��õ���ɫ������Һ����2������ɫ������Һ�м����Ȼ�����Һ���а�ɫ�������ɡ���3�����ɫ�����м���������ϡ���ᣬ�в��ֳ����ܽ⣬��������ʹ����ʯ��ˮ����ǵ����塣����������ʵ���ð�ɫ��ĩ��һ������________�����ܺ���_______��һ��û��_________��
һ������: Na2SO4��Na2CO3�����ܺ���: NaCl��һ��û��: CuSO4��AgCl
���ݣ�1���й�����ȫ�ܽ⣬��AgCl������ˮ����ԭ��ĩ��һ��û��AgCl��ͬʱ�õ���ɫ��Һ����CuSO4��Һ����ɫ����ԭ��ĩ��һ��û��CuSO4���ٸ��ݣ�2���м��Ȼ���������ɫ����������Һ�в����ڱ����ӣ���ԭ��ĩ����Na2SO4����Na2CO3���ٸ��ݣ�3����ɫ�������в��ֳ����ܽⲢ�ų����壬�����Ϊ̼�ᱵ��������ԭ��ĩ��һ����Na2CO3�������ᱵ�������ᣬ��ԭ��ĩ��һ����Na2SO4��

��ϰ��ϵ�д�
 Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
�����Ŀ
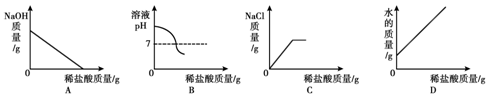
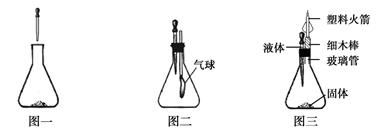

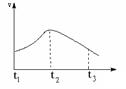
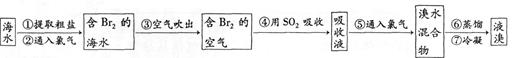
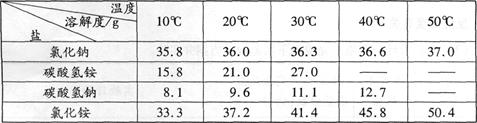
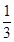 ����ҵ�ϳ��á������������壬�乤���������£�
����ҵ�ϳ��á������������壬�乤���������£�