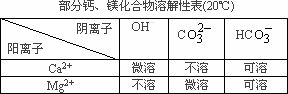
��Ŀ����
��25 gʯ��ʯ����Ҫ�ɷ���CaCO3������73 gϡ�����У�ʯ��ʯ�е����ʲ������ᷴӦ��Ҳ������ˮ��ǡ����ȫ��Ӧ������8.8 g������̼���壮������1��ʯ��ʯ��̼��Ƶ�����������
��2��ϡ���������ʵ�����������
��3����Ӧ����Һ�����ʵ�����������
������չ��Ҫ����50 g���ʵ���������Ϊ20%���������Һ�����ṩ25 g���ʵ���������Ϊ40%���������Һ��20 g���ʵ���������Ϊ15%���������Һ���㹻�������ؾ��������ˮ����ѡ��������ҩƷ������������Ʒ��������±���
| ���Ʒ�����ֻҪ˵������ʱ����ĸ���ҩƷ�������� | |
| ����һ | |
| ������ | |
| ������ |
���������㣺�����������������ʯ��ʯ��������ϡ���������������̼��������ᷴӦ�Ļ�ѧ����ʽ���Լ����ʯ��ʯ��̼��Ƶ�����������ϡ���������ʵ����������������Ȼ��Ƶ������������������Ӧ����Һ�����ʵ�����������
������չ����������ع����ˮ�ܽ���ɣ�����40%���������Һ��ˮϡ�Ͷ��ɣ�����15%�������Һ�м�����ع����ˮ�ܽ���ɵȣ��������ɣ���
������չ����������ع����ˮ�ܽ���ɣ�����40%���������Һ��ˮϡ�Ͷ��ɣ�����15%�������Һ�м�����ع����ˮ�ܽ���ɵȣ��������ɣ���
����⣺��ʯ��ʯ��̼��Ƶ���������Ϊx��ϡ���������ʵ���������Ϊy�������Ȼ��Ƶ�����Ϊz��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 111 44
25g��x 73g��y z 8.8g
��1��
=
��x=80%
��2��
=
��y=20%
��3��
=
��z=22.2g
��Ӧ����Һ�����ʵ���������Ϊ
��100%��26.4%
�𣺣�1��ʯ��ʯ��̼��Ƶ���������Ϊ80%��
��2��ϡ���������ʵ���������Ϊ20%��
��3����Ӧ����Һ�����ʵ���������Ϊ26.4%��
������չ��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 111 44
25g��x 73g��y z 8.8g
��1��
| 100 |
| 44 |
| 25g��x |
| 8.8g |
��2��
| 73 |
| 44 |
| 73g��y |
| 8.8g |
��3��
| 111 |
| 44 |
| z |
| 8.8g |
��Ӧ����Һ�����ʵ���������Ϊ
| 22.2g |
| 25g��80%+73g-8.8g |
�𣺣�1��ʯ��ʯ��̼��Ƶ���������Ϊ80%��
��2��ϡ���������ʵ���������Ϊ20%��
��3����Ӧ����Һ�����ʵ���������Ϊ26.4%��
������չ��
| ���Ʒ��� | |
| ����һ | 10g����ع��壬40gˮ |
| ������ | 20g40%���������Һ��25gˮ |
| ������ | 20g15%���������Һ��7g����ع��壬23gˮ |
������������Ҫ���麬�������ʵĻ�ѧ����ʽ������������������ļ��㣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
��25 gʯ��ʯ����Ҫ�ɷ���CaCO3������73 gϡ�����У�ʯ��ʯ�е����ʲ������ᷴӦ��Ҳ������ˮ��ǡ����ȫ��Ӧ������8.8 g������̼���壮����
��1��ʯ��ʯ��̼��Ƶ�����������
��2��ϡ���������ʵ�����������
��3����Ӧ����Һ�����ʵ�����������
������չ��Ҫ����50 g���ʵ���������Ϊ20%���������Һ�����ṩ25 g���ʵ���������Ϊ40%���������Һ��20 g���ʵ���������Ϊ15%���������Һ���㹻�������ؾ��������ˮ����ѡ��������ҩƷ������������Ʒ��������±���
| ���Ʒ�����ֻҪ˵������ʱ����ĸ���ҩƷ�������� | |
| ����һ | |
| ������ | |
| ������ |
��25 gʯ��ʯ����Ҫ�ɷ���CaCO3������73 gϡ�����У�ʯ��ʯ�е����ʲ������ᷴӦ��Ҳ������ˮ��ǡ����ȫ��Ӧ������8.8 g������̼���壮����
��1��ʯ��ʯ��̼��Ƶ�����������
��2��ϡ���������ʵ�����������
��3����Ӧ����Һ�����ʵ�����������
������չ��Ҫ����50 g���ʵ���������Ϊ20%���������Һ�����ṩ25 g���ʵ���������Ϊ40%���������Һ��20 g���ʵ���������Ϊ15%���������Һ���㹻�������ؾ��������ˮ����ѡ��������ҩƷ������������Ʒ��������±���
��1��ʯ��ʯ��̼��Ƶ�����������
��2��ϡ���������ʵ�����������
��3����Ӧ����Һ�����ʵ�����������
������չ��Ҫ����50 g���ʵ���������Ϊ20%���������Һ�����ṩ25 g���ʵ���������Ϊ40%���������Һ��20 g���ʵ���������Ϊ15%���������Һ���㹻�������ؾ��������ˮ����ѡ��������ҩƷ������������Ʒ��������±���
| ���Ʒ�����ֻҪ˵������ʱ����ĸ���ҩƷ�������� | |
| ����һ | |
| ������ | |
| ������ |