��Ŀ����
��25 gʯ��ʯ����Ҫ�ɷ���CaCO3������73 gϡ�����У�ʯ��ʯ�е����ʲ������ᷴӦ��Ҳ������ˮ��ǡ����ȫ��Ӧ������8.8 g������̼���壮����
��1��ʯ��ʯ��̼��Ƶ�����������
��2��ϡ���������ʵ�����������
��3����Ӧ����Һ�����ʵ�����������
������չ��Ҫ����50 g���ʵ���������Ϊ20%���������Һ�����ṩ25 g���ʵ���������Ϊ40%���������Һ��20 g���ʵ���������Ϊ15%���������Һ���㹻�������ؾ��������ˮ����ѡ��������ҩƷ������������Ʒ��������±���
| ���Ʒ�����ֻҪ˵������ʱ����ĸ���ҩƷ�������� | |
| ����һ | |
| ������ | |
| ������ |
�⣺��ʯ��ʯ��̼��Ƶ���������Ϊx��ϡ���������ʵ���������Ϊy�������Ȼ��Ƶ�����Ϊz��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 111 44
25g��x 73g��y z 8.8g
��1�� =
= ��x=80%
��x=80%
��2�� =
=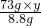 ��y=20%
��y=20%
��3�� =
=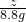 ��z=22.2g
��z=22.2g
��Ӧ����Һ�����ʵ���������Ϊ
 ��100%��26.4%
��100%��26.4%
�𣺣�1��ʯ��ʯ��̼��Ƶ���������Ϊ80%��
��2��ϡ���������ʵ���������Ϊ20%��
��3����Ӧ����Һ�����ʵ���������Ϊ26.4%��
������չ��
���������㣺�����������������ʯ��ʯ��������ϡ���������������̼��������ᷴӦ�Ļ�ѧ����ʽ���Լ����ʯ��ʯ��̼��Ƶ�����������ϡ���������ʵ����������������Ȼ��Ƶ������������������Ӧ����Һ�����ʵ�����������
������չ����������ع����ˮ�ܽ���ɣ�����40%���������Һ��ˮϡ�Ͷ��ɣ�����15%�������Һ�м�����ع����ˮ�ܽ���ɵȣ��������ɣ���
������������Ҫ���麬�������ʵĻ�ѧ����ʽ������������������ļ��㣬�ѶȽϴ�
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 111 44
25g��x 73g��y z 8.8g
��1��
 =
= ��x=80%
��x=80%��2��
 =
=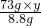 ��y=20%
��y=20%��3��
 =
=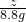 ��z=22.2g
��z=22.2g��Ӧ����Һ�����ʵ���������Ϊ
 ��100%��26.4%
��100%��26.4%�𣺣�1��ʯ��ʯ��̼��Ƶ���������Ϊ80%��
��2��ϡ���������ʵ���������Ϊ20%��
��3����Ӧ����Һ�����ʵ���������Ϊ26.4%��
������չ��
| ���Ʒ��� | |
| ����һ | 10g����ع��壬40gˮ |
| ������ | 20g40%���������Һ��25gˮ |
| ������ | 20g15%���������Һ��7g����ع��壬23gˮ |
���������㣺�����������������ʯ��ʯ��������ϡ���������������̼��������ᷴӦ�Ļ�ѧ����ʽ���Լ����ʯ��ʯ��̼��Ƶ�����������ϡ���������ʵ����������������Ȼ��Ƶ������������������Ӧ����Һ�����ʵ�����������
������չ����������ع����ˮ�ܽ���ɣ�����40%���������Һ��ˮϡ�Ͷ��ɣ�����15%�������Һ�м�����ع����ˮ�ܽ���ɵȣ��������ɣ���
������������Ҫ���麬�������ʵĻ�ѧ����ʽ������������������ļ��㣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ


 ________��
________�� ________��
________�� ________��
________��