��Ŀ����
����Ŀ��ѧϰ����ͼ��֪ʶ��С��֪���˰״���Ҫ�ɷ��Ǵ��ᣨCH3COOH����Ϊ�˲ⶨ�״��д������������������4%��NaOH��Һ�μӵ�30 g�״��У�����Ӧǡ����ȫ��Ӧʱ���ٶ������ɷֲ��μӷ�Ӧ��������ȥNaOH��Һ25 g��
��1����֪��Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+NaOH=CH3COONa+H2O���÷�Ӧ�������� ��
��2�������״��д��������������
���𰸡��кͷ�Ӧ���ֽⷴӦ����5%��
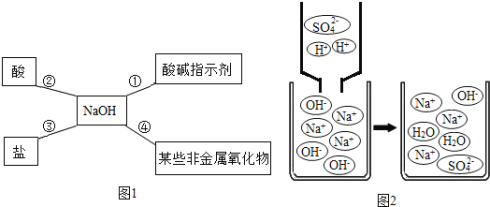
����������1����Ӧ��CH3COOHΪ�ᡢNaOHΪ���ͼ���кͷ�Ӧ���кͷ�Ӧ�Ǹ��ֽⷴӦ�е�һ�����ⷴӦ��
��2����״��д������������Ϊx��
CH3COOH+NaOH=CH3COONa+H2O
60 40
30g��x 25g��4%
60��40=��30g��x������25g��4%��
��֮��![]()
�𣺰״��д������������5%��

��ϰ��ϵ�д�
�����Ŀ