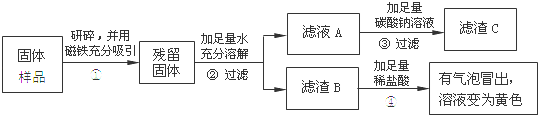
��Ŀ����
������ȫȼ������CO2��H2O��ȼ��ʱ�����Ϊ���㣬���������¶Ƚϵͣ����ᷢ������ȫȼ�գ�������CO2��H2O�⣬�����������Сϣ�Դ˽�����̽����
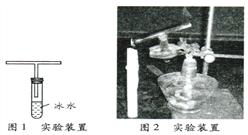
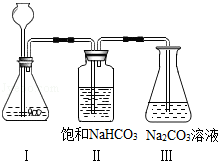
��ͼ1��ʾװ�ù̶�������̨�ϣ���ȼ�������ܵ�һ�˲������IJ��֣����������еIJ������壨��ͼ2����ʵ���пɹ۲쵽��
��1��ʢ�б�ˮ�Թܵ�ˮ������һ�㱡���İ�ɫ���壬˵�������������к��� ��
��2���������ĵĵ��ܱ�ڣ�˵��������ȫȼ��ʱ�� ���ѧʽ�����ɡ�
��3����һ�����С�ձ����ڵ�����һ�ˣ��������ڱ��� ��˵������ȼ����H2O���ɡ���ȼ�ƾ��ƣ����ֵ��������ȼ�գ��ڻ����Ϸ�����մ��ʯ��ˮ���ձ������ǣ� ����ܡ����ܡ���֤���õ�������ȼ��һ��������CO2�������� ������ţ���
��CO2��ʹ����ʯ��ˮ�����
��ʯ��������ʹ����ʯ��ˮ�����
������ȼ�����ɵ�CO2Ҳ��ʹ����ʯ��ˮ�����
��4����ʵ��֤���õ������廹����CO��COȼ�յIJ���������ȼ�ղ���֮һ��ͬ�����ڿ�����ȼ�յĻ�ѧ��ӦʽΪ ��

��ͼ1��ʾװ�ù̶�������̨�ϣ���ȼ�������ܵ�һ�˲������IJ��֣����������еIJ������壨��ͼ2����ʵ���пɹ۲쵽��
��1��ʢ�б�ˮ�Թܵ�ˮ������һ�㱡���İ�ɫ���壬˵�������������к��� ��
��2���������ĵĵ��ܱ�ڣ�˵��������ȫȼ��ʱ�� ���ѧʽ�����ɡ�
��3����һ�����С�ձ����ڵ�����һ�ˣ��������ڱ��� ��˵������ȼ����H2O���ɡ���ȼ�ƾ��ƣ����ֵ��������ȼ�գ��ڻ����Ϸ�����մ��ʯ��ˮ���ձ������ǣ� ����ܡ����ܡ���֤���õ�������ȼ��һ��������CO2�������� ������ţ���
��CO2��ʹ����ʯ��ˮ�����
��ʯ��������ʹ����ʯ��ˮ�����
������ȼ�����ɵ�CO2Ҳ��ʹ����ʯ��ˮ�����
��4����ʵ��֤���õ������廹����CO��COȼ�յIJ���������ȼ�ղ���֮һ��ͬ�����ڿ�����ȼ�յĻ�ѧ��ӦʽΪ ��
��1��ʯ������ ��2��C ��3��ˮ������ˮ�飩 ���� �� ��3��CO+O2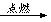 CO2
CO2
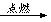 CO2
CO2�����������1��ʢ�б�ˮ�Թܵ�ˮ������һ�㱡���İ�ɫ������ʯ���������̳�ʯ�����壬��˵�������������к���ʯ����������2���������ĵĵ��ܱ�ڣ�˵��������ȫȼ��ʱ����̼���ʻ�ѧʽΪC����3����һ�����С�ձ����ڵ�����һ�ˣ��������ڱ���ˮ������ˮ�飩��˵������ȼ����H2O���ɣ���ȼ�ƾ��ƣ����ֵ��������ȼ�գ��ڻ����Ϸ�����մ��ʯ��ˮ���ձ������ǣ�����֤���õ�������ȼ��һ��������CO2������������ȼ�����ɵ�CO2Ҳ��ʹ����ʯ��ˮ����ǣ���4��һ����̼�ڿ�����ȼ�յĻ�ѧ��ӦʽΪ��CO+O2
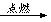 CO2��
CO2��
��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д�
�����Ŀ