题目内容
根据以下实验装置图,回答有关问题:
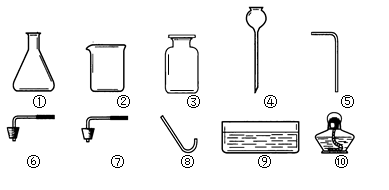
(1)写出标号仪器的名称:① ②
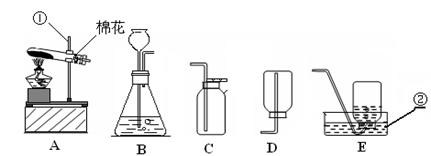
(2)彬彬用A、E组合装置制取氧气,小林说还能用A和C组合,理由是__________________;写出反应的文字或符号表达式为 ________________________ ,试管口放一团棉花的目的是 _____________________。若用过氧化氢溶液与二氧化锰制取氧气,所选用的发生装置是 _______(填编号);其中二氧化锰在反应中起________作用。
(3)彬彬实验的过程中试管出现了破裂,则可能是因为①_____________ ____、② 而导致的(至少写两点)。
(4)彬彬在收集的氧气中做铁丝燃烧实验时,预先在集气瓶底部放少量水,目的是 。
(5)实验室用高锰酸钾来制取氧气。经探究,生成氧气的质量约是高锰酸钾质量的1/10。回答下列问题,
①现有高锰酸钾32 g,大约能生成氧气的质量是多少?写出计算过程:
②这些氧气相当于多少升的空气中所含有的氧气(结果取整数)?(氧气在该条件下密度为1.43 g/L)

(1)写出标号仪器的名称:① ②
(2)彬彬用A、E组合装置制取氧气,小林说还能用A和C组合,理由是__________________;写出反应的文字或符号表达式为 ________________________ ,试管口放一团棉花的目的是 _____________________。若用过氧化氢溶液与二氧化锰制取氧气,所选用的发生装置是 _______(填编号);其中二氧化锰在反应中起________作用。
(3)彬彬实验的过程中试管出现了破裂,则可能是因为①_____________ ____、② 而导致的(至少写两点)。
(4)彬彬在收集的氧气中做铁丝燃烧实验时,预先在集气瓶底部放少量水,目的是 。
(5)实验室用高锰酸钾来制取氧气。经探究,生成氧气的质量约是高锰酸钾质量的1/10。回答下列问题,
①现有高锰酸钾32 g,大约能生成氧气的质量是多少?写出计算过程:
②这些氧气相当于多少升的空气中所含有的氧气(结果取整数)?(氧气在该条件下密度为1.43 g/L)
(1):① 铁架台 ② 水槽
(2)氧气密度比空气略大;2KMnO4 K2MnO4 +MnO2+ O2↑,
K2MnO4 +MnO2+ O2↑,
防止高锰酸钾粉末进入导管,堵塞导管。 B ;催化
(3)①加热不均匀,局部过热 ②加热产生的水蒸气倒流。
(4)防止生成的熔化物溅落,炸裂集气瓶
(5)① 3.2g ② 11L
(2)氧气密度比空气略大;2KMnO4
 K2MnO4 +MnO2+ O2↑,
K2MnO4 +MnO2+ O2↑,防止高锰酸钾粉末进入导管,堵塞导管。 B ;催化
(3)①加热不均匀,局部过热 ②加热产生的水蒸气倒流。
(4)防止生成的熔化物溅落,炸裂集气瓶
(5)① 3.2g ② 11L
(1)由图中所指①为酒精灯,②为水槽;
(2)氧气密度比空气略大,所以可选择向上排空气法收集,高锰酸钾在加热的条件下生成锰酸钾、二氧化锰和氧气,试管口放一团棉花的目的是防止高锰酸钾粉末进入导管,堵塞导管,过氧化氢溶液与二氧化锰反应制取氧气是“固液常温型”,所以选择B装置,二氧化锰在反应中加速了化学反应速率,而本身的质量和化学性质在反应前后没有发生变化,起催化作用.
(3)试管破裂的原因:①加热前没有给试管预热,受热不均匀;②试管外壁有水,加热时没擦干;③试管接触到灯芯;④试管口部高于试管底部,导致冷凝水倒流炸裂试管;④实验完毕,先熄灭酒精灯,后移开导管等;
(4)做铁丝燃烧实验时,集气瓶底部放少量水的目的是防止反应后生成的熔化物溅落,炸裂集气瓶底;
(5)生成氧气的质量约是高锰酸钾质量的1/10,由高锰酸钾的质量是32g,可知氧气的质量是32g×1/10 =3.2g;由氧气的质量可以算出其体积,又因为氧气大约占了空气总体积的21%,所以空气的体积为:3.2g÷1.43g/L÷21%≈11L.
(2)氧气密度比空气略大,所以可选择向上排空气法收集,高锰酸钾在加热的条件下生成锰酸钾、二氧化锰和氧气,试管口放一团棉花的目的是防止高锰酸钾粉末进入导管,堵塞导管,过氧化氢溶液与二氧化锰反应制取氧气是“固液常温型”,所以选择B装置,二氧化锰在反应中加速了化学反应速率,而本身的质量和化学性质在反应前后没有发生变化,起催化作用.
(3)试管破裂的原因:①加热前没有给试管预热,受热不均匀;②试管外壁有水,加热时没擦干;③试管接触到灯芯;④试管口部高于试管底部,导致冷凝水倒流炸裂试管;④实验完毕,先熄灭酒精灯,后移开导管等;
(4)做铁丝燃烧实验时,集气瓶底部放少量水的目的是防止反应后生成的熔化物溅落,炸裂集气瓶底;
(5)生成氧气的质量约是高锰酸钾质量的1/10,由高锰酸钾的质量是32g,可知氧气的质量是32g×1/10 =3.2g;由氧气的质量可以算出其体积,又因为氧气大约占了空气总体积的21%,所以空气的体积为:3.2g÷1.43g/L÷21%≈11L.

练习册系列答案
 名题金卷系列答案
名题金卷系列答案 优加精卷系列答案
优加精卷系列答案
相关题目