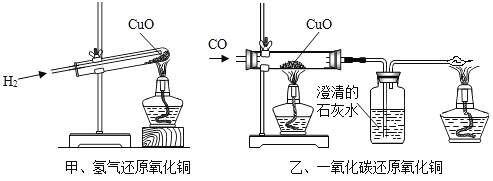
��Ŀ����
��ʽ����������ѧʽΪ��NiOOH�������������ص��������ϡ���ҵ����ij��Һ����Ҫ����Ϊ�����������仯ѧʽΪ��NiSO4���������һЩ�������ʡ���Ϊԭ������NiOOH �IJ������£�
���� I�����Һ�м��� Na2CO3 ��Һ���ɳ��������ˣ�ϴ�ӣ��õ����� NiCO3��
���� II������� NiCO3 �м���ϡ���ᣬ�����ܽ⣬�õ���������Һ��
���� III��������������Һ�� pH,�ɽ�������ת��Ϊ����������������ѧʽΪ��Ni(OH)2���� ���˲�ϴ�ӳ�����
���� IV�������������������ڿ����м��ȣ����ɼ�ʽ����������ѧʽΪ��NiOOH���� ��ش��������⣺
��1��NiOOH �� Ni �Ļ��ϼ�Ϊ��_____��
��2��д�����Һ�м��� Na2CO3 ��Һ���� NiCO3 �����Ļ�ѧ����ʽ_____��
��3������ I �Ͳ��� II �������ǣ�_____��
��4������ IV�������˼�ʽ��������ˮ����д������ IV�з�����Ӧ�Ļ�ѧ����ʽ��______��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���ѧС��ȡ��״�ʹ�С����ͬ��þƬ��пƬ����Ƭ����Ƭ���ֱ���5%��ϡ���ᷴӦ����÷�Ӧ�ٶ��ɴ�С��˳���ǣ�þ>п>��>����Ϊʲôʵ������������˳����ȫһ���أ�С��ͬѧ������ܵ�ԭ�����������̽��ʵ�顣(˵����ʵ�����������������������)
ʵ����� | ���� | ��Ӧ�ٶ� | |
�� | ȡ��״�ʹ�С������ͬ��þƬ����Ƭ����Ƭ��пƬ��________�������Թ��У��ֱ�ͬʱ���� | 5%��ϡ���� | þ��п�������� |
�� | 10%��ϡ���� | þ��п�������� | |
�� | 5%��ϡ���� | þ������п���� | |
�� | 10%��ϡ���� | þ������п���� |
(1)����ʵ���к��ߴ��IJ�����__________�����ʵ����Ŀ����____________________��
(2)�Ա�ʵ��_____(�����)�������ж������ᷴӦ���ٶ��������г��ֱ仯��ԭ�������_____�йأ�ijͬѧ�����벻ͬŨ��ϡ���ᷴӦ���Թ��м�������Ȼ��ƹ��壬���ַ�Ӧ�ٶȾ����Ա�죬���۽Ƕȷ�����ԭ����_______________��
(3)ʵ���ͬѧ�ǻ����˱���̽��ʵ��������ϡ���ᷴӦ������ͼ��������ȷ����_____(����ĸ���)���ж�����Ϊ____________________��
A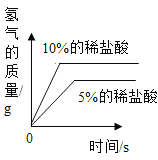 B
B 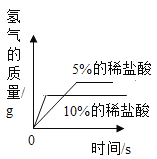 C
C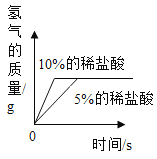
��������ѧʽΪ��Cl2����һ�ֻ���ɫ���壬������ˮ��õ�����Һ��Ϊ��ˮ����������ˮʱ��������������ˮ�������·�Ӧ��Cl2+H2O��HCl+HClO����ˮ��dz����ɫ�� ��ˮ���ж������ʣ�������ж������ʡ���֪�������ᣨ��ѧʽΪ��HClO������ǿ���� �ԣ��ܹ�����ɫ���ʣ������ָʾ��������Ϊ��ɫ���ʣ��������Ժ����������뺬 CO3 ���η�Ӧ����Ԥ����ˮ�Ļ�ѧ���ʣ�˵��Ԥ������ݣ������ʵ����֤����ѡ�Լ���Na2SO4 ��Һ��CaCO3 ���塢AgNO3 ��Һ����ɫʯ����Һ��BaCl2 ��Һ��þ�ۡ�ͭƬ��
Ԥ�� | Ԥ������� | ��֤Ԥ���ʵ�������Ԥ������ |
�� �ܹ��뺬Ag+�Ŀ������η�Ӧ | ��ˮ�к���Cl-,AgCl������ˮ�� | ȡ������ˮ���Թ��У�����۲쵽��_______��Ԥ����� |
�� �ܹ���_______����������𣩷�Ӧ | _____ | ����۲쵽��_______��Ԥ����� |
�� �ܹ���_______����������𣩷�Ӧ | _____ | ����۲쵽��_______��Ԥ����� |


 �dz��л�ѧ����������,����֮���ת����ϵ��ͼ��ʾ����֪F��������Ҫ�Ĺ�������,���ַ�Ӧ������ʡ�ԡ���ش��������⣺
�dz��л�ѧ����������,����֮���ת����ϵ��ͼ��ʾ����֪F��������Ҫ�Ĺ�������,���ַ�Ӧ������ʡ�ԡ���ش��������⣺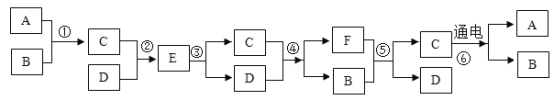
 дһ������
дһ������ ��______________��
��______________�� �Ļ�ѧ����ʽ��______________��
�Ļ�ѧ����ʽ��______________��