��Ŀ����
 ijͬѧ����˲ⶨ����������������ʵ�飬ʵ��װ����ͼ����ͬѧ��ʵ�鲽�����£�
ijͬѧ����˲ⶨ����������������ʵ�飬ʵ��װ����ͼ����ͬѧ��ʵ�鲽�����£��ٽ�ͼ�еļ���ƿ��Ϊ5�ȷݣ������ñ�ǣ�
���ڴ���Ƥ���͵��ܵ�ȼ�ճ���װ�������ĺ��ף��������ϵ�ֹˮ�мн����ھƾ����ϵ�ȼ���ף����������뼯��ƿ�ڣ�������Ƥ����
�۳�ַ�Ӧ������ƿ��ȴ�����£���ֹˮ�У�
��ش��������⣺
��1����ʵ���к������Թ�����Ŀ����
��2���۲쵽��������
��3���÷�Ӧ�����ֱ���ʽΪ
��4����ʵ������۳�����
��5������������̿�ۣ���ʵ���ܷ��óɹ���Ϊʲô��
��6��ʵ�������ֲ����ֵ��ʵ�ʿ����������ĺ���С��������������ҳ�ʵ������ԭ��
���㣺������ɵIJⶨ,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺������ˮ
��������1����Ϊ��ʹ����ƿ�ڵ�������ȫ��Ӧ��Ҫ��������ĺ���ȥ������𣻹ʴ�Ϊ��ʹ����ƿ�е�������ȫ��Ӧ��
��2���Ӻ���ȼ�����Ŀ����е�������ʹ�����ƿ�����������С��ѹǿ��С���Ӷ����ⲿ����ѹ��������ʹ�ձ��ڵ�ˮ���뼯��ƿ��ͨ���������뼯��ƿ��ˮ������ȷ���������������������������������Լռ���������
ȥ�������
��3���Ӻ��Ϳ����е������ڵ�ȼ�������·�Ӧ��������������ȥ�������
��4���Ӽ���ƿ��ʣ���������Ҫ�ǵ���������û�м���ȼ�գ�˵����������֧��ȼ�գ����Ļ�ѧ���ʲ����ã����ձ��ڵ�ˮ���뼯��ƿ��ˮû�г�����������ƿȥ�������
��5����ľ̿ȼ��ʱ����ȻҲ���Ŀ�������������������ͬ������Ķ�����̼���壬�����˼���ƿ�����û�м�С���ձ��ڵ�ˮ������뼯��ƿ������ʵ��ʧ��ȥ�������
��6����������ú��������㣬���ƿ�ڿ����е�����û����ȫ�����ģ�ƿ�ڲ��������л������������Ի�ʹ����õ��������������ƫС�����װ�õ������Բ��ã�����������뼯��ƿ�У��γɲ���ѹǿ������ˮ���뼯��ƿ�������С���������õ��������������ƫСȥ�������
��2���Ӻ���ȼ�����Ŀ����е�������ʹ�����ƿ�����������С��ѹǿ��С���Ӷ����ⲿ����ѹ��������ʹ�ձ��ڵ�ˮ���뼯��ƿ��ͨ���������뼯��ƿ��ˮ������ȷ���������������������������������Լռ���������
| 1 |
| 5 |
��3���Ӻ��Ϳ����е������ڵ�ȼ�������·�Ӧ��������������ȥ�������
��4���Ӽ���ƿ��ʣ���������Ҫ�ǵ���������û�м���ȼ�գ�˵����������֧��ȼ�գ����Ļ�ѧ���ʲ����ã����ձ��ڵ�ˮ���뼯��ƿ��ˮû�г�����������ƿȥ�������
��5����ľ̿ȼ��ʱ����ȻҲ���Ŀ�������������������ͬ������Ķ�����̼���壬�����˼���ƿ�����û�м�С���ձ��ڵ�ˮ������뼯��ƿ������ʵ��ʧ��ȥ�������
��6����������ú��������㣬���ƿ�ڿ����е�����û����ȫ�����ģ�ƿ�ڲ��������л������������Ի�ʹ����õ��������������ƫС�����װ�õ������Բ��ã�����������뼯��ƿ�У��γɲ���ѹǿ������ˮ���뼯��ƿ�������С���������õ��������������ƫСȥ�������
����⣺��1��Ϊ��ʹ����ƿ�ڵ�������ȫ��Ӧ��Ҫ��������ĺ��ף��ʴ�Ϊ��ʹ����ƿ�е�������ȫ��Ӧ��
��2������ȼ�����Ŀ����е�������ʹ�����ƿ�����������С��ѹǿ��С���Ӷ����ⲿ����ѹ��������ʹ�ձ��ڵ�ˮ���뼯��ƿ��ͨ���������뼯��ƿ��ˮ������ȷ���������������������������������Լռ���������
�������ձ��е�ˮ���н��뼯��ƿ���Ҵﵽ����ƿ�ݻ���
���ʴ�Ϊ���ձ��е�ˮ���н��뼯��ƿ���Ҵﵽ����ƿ�ݻ���
��
��3�����Ϳ����е������ڵ�ȼ�������·�Ӧ�������������ף��䷴Ӧ�����ֱ���ʽΪ����+����
���������ף��ʴ�Ϊ����+����
���������ף�
��4������ƿ��ʣ���������Ҫ�ǵ���������û�м���ȼ�գ�˵����������֧��ȼ�գ����Ļ�ѧ���ʲ����ã����ձ��ڵ�ˮ���뼯��ƿ��ˮû�г�����������ƿ��˵������������ˮ���ʴ�Ϊ���� �����ã�
��5������ľ̿ȼ��ʱ����ȻҲ���Ŀ�������������������ͬ������Ķ�����̼���壬�����˼���ƿ�����û�м�С���ձ��ڵ�ˮ������뼯��ƿ������ʵ��ʧ�ܣ�
�ʴ�Ϊ������ ����ľ̿ȼ��ʱ����ȻҲ���Ŀ�������������������ͬ������Ķ�����̼���壬�����˼���ƿ�����û�м�С���ձ��ڵ�ˮ������뼯��ƿ��
��6��������ú��������㣬���ƿ�ڿ����е�����û����ȫ�����ģ�ƿ�ڲ��������л������������Ի�ʹ����õ��������������ƫС�����װ�õ������Բ��ã�����������뼯��ƿ�У��γɲ���ѹǿ������ˮ���뼯��ƿ�������С���������õ��������������ƫС���ʴ�Ϊ������������ װ��©����
��2������ȼ�����Ŀ����е�������ʹ�����ƿ�����������С��ѹǿ��С���Ӷ����ⲿ����ѹ��������ʹ�ձ��ڵ�ˮ���뼯��ƿ��ͨ���������뼯��ƿ��ˮ������ȷ���������������������������������Լռ���������
| 1 |
| 5 |
| 1 |
| 5 |
| 1 |
| 5 |
| 1 |
| 5 |
��3�����Ϳ����е������ڵ�ȼ�������·�Ӧ�������������ף��䷴Ӧ�����ֱ���ʽΪ����+����
| ��ȼ |
| ��ȼ |
��4������ƿ��ʣ���������Ҫ�ǵ���������û�м���ȼ�գ�˵����������֧��ȼ�գ����Ļ�ѧ���ʲ����ã����ձ��ڵ�ˮ���뼯��ƿ��ˮû�г�����������ƿ��˵������������ˮ���ʴ�Ϊ���� �����ã�
��5������ľ̿ȼ��ʱ����ȻҲ���Ŀ�������������������ͬ������Ķ�����̼���壬�����˼���ƿ�����û�м�С���ձ��ڵ�ˮ������뼯��ƿ������ʵ��ʧ�ܣ�
�ʴ�Ϊ������ ����ľ̿ȼ��ʱ����ȻҲ���Ŀ�������������������ͬ������Ķ�����̼���壬�����˼���ƿ�����û�м�С���ձ��ڵ�ˮ������뼯��ƿ��
��6��������ú��������㣬���ƿ�ڿ����е�����û����ȫ�����ģ�ƿ�ڲ��������л������������Ի�ʹ����õ��������������ƫС�����װ�õ������Բ��ã�����������뼯��ƿ�У��γɲ���ѹǿ������ˮ���뼯��ƿ�������С���������õ��������������ƫС���ʴ�Ϊ������������ װ��©����
��������ס�ⶨ����������������ʵ��ԭ�������ú���ȼ�����Ŀ����е�������ʹ�����ƿ�����������С��ѹǿ��С���Ӷ����ⲿ����ѹ��������ʹ�ձ��ڵ�ˮ���뼯��ƿ��ͨ���������뼯��ƿ��ˮ������ȷ�����������������������

��ϰ��ϵ�д�
 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
�����Ŀ
����͵���Ҫ�ɷ�֮һ��ά����D3�����Ļ�ѧʽΪC27H44O�������й�˵����ȷ���ǣ�������
| A��ά����D3����Է�������Ϊ384g |
| B��ά����D3������Ԫ����� |
| C��ά����D3��̼����Ԫ�ص�������Ϊ27��44 |
| D��ά����D3��27��̼ԭ�ӡ�44����ԭ�Ӻ�1����ԭ�ӹ��� |

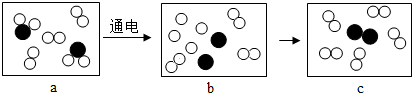
 ���͡��𡱷ֱ��ʾ��ԭ�Ӻ���ԭ�ӣ�a��c�ֱ��ʾ��Ӧǰ�ͷ�Ӧ������ʣ���ش�
���͡��𡱷ֱ��ʾ��ԭ�Ӻ���ԭ�ӣ�a��c�ֱ��ʾ��Ӧǰ�ͷ�Ӧ������ʣ���ش�