��Ŀ����
����Ŀ����ѧ������������ϢϢ��أ������û�ѧ֪ʶ�ش���������:
��1�������������ںϳɲ��ϵ���_____________������ĸ��
A ����ϩB ��C �ϳ���D ɣ��˿
��2����Щ��ׯ����ȡ�õ���ˮ������_____________���ֵ���ˮ����ˮ����Ӳˮ��������Ϊ����ˮ��Ӳ�Ȳ�ɱ��ˮ�в�ԭ����ɲ��õķ�����______________��
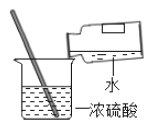
��3���ᳫ����ú��______________����Ȼ������ʯȼ�ϣ������ڸ��ƿ���������
��4���¹ڷ��������ڼ䣬84����Һ�dz�������������
��84����Һ�ͽ������ʹ��ʱ�ᷢ����ѧ��Ӧ2 HCl+NaClO= NaCl+H2O+X��X�����ж�����˶��߲��ܻ��ʹ�á���ôX�Ļ�ѧʽΪ________��
�ڽ�������������Һ�����ڳ�������Һ��ζ��˵�����Ӿ��е�����Ϊ_________��
��5����ʯ����������������;�㷺���ȿ�����________���ѧʽ����һ�����������Ʊ�������Һ���ֿ�����������_____________����������������������������������������
��6�������������ߵĽ������ϡ�ij������ÿ��������3500t��Fe2O380%�ij�����ʯ���ó������Ͽ��ղ���Fe98%��������������__________t��
���𰸡�AC ����ˮ ��� ʯ�� C12 �����ڲ����˶� CuSO4 ���� 2000
��������
��1���ϳɲ��ϰ������ϳ���ά���ϳ������ϡ�
A������ϩ�����ϣ����ںϳɲ��ϣ�
B��������Ȼ���ʣ������ںϳɲ��ϣ�
C���ϳ������ںϳɲ��ϣ�
D��ɣ��˿����Ȼ���ʣ������ںϳ���ά��
��ѡ��AC��
��2���ѷ���ˮ�ֱ�μӵ�ʢ�е�������ˮ��Ӳˮ���ձ��У����裬�۲��ձ��в�����ĭ���������ĭ�������ˮ����ĭ�١����������Ӳˮ�������÷���ˮ��������Ӳˮ����ˮ�������г��ü�����еķ�������ˮ��Ӳ�ȡ�
�������ˮ����С�
��3������ʯȼ����ָ��ú��ʯ�͡���Ȼ����
���ʯ�͡�
��4�����ɷ�Ӧ�Ļ�ѧ����ʽNaClO+2HCl�TNaCl+H2O+X����֪����Ӧ�����ơ��ȡ��⡢��ԭ�Ӹ����ֱ�Ϊ1��3��2��1����Ӧ������������ơ��ȡ��⡢��ԭ�Ӹ����ֱ�Ϊ1��1��2��1�����ݻ�ѧ��Ӧǰ��ԭ�����ࡢ��Ŀ���䣬��ÿ��X������2����ԭ�ӹ��ɣ�������X�Ļ�ѧʽΪCl2��
���Cl2��
�ڽ�������������Һ�����ڳ�������Һ��ζ��˵�����Ӿ��е�����Ϊ�������ڲ����˶���
��������ڲ����˶���
��5��ũҵ�ϣ���ʯ�ҿ�������ͭ��һ����������Ʊ�������Һ�����е����������Լ��ԣ�����������������������
���CuSO4�����ԡ�
��6����ó������Ͽ��ղ�����98%��������������x�����У�

���x=2000t��
���2000��

 ��ĩ�����ϵ�д�
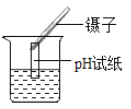
��ĩ�����ϵ�д�����Ŀ��ijͬѧ������ͼ����ʵ�顣

��l����ʵ���з�Ӧ�Ļ�ѧ����ʽΪ____________��
��2����ʵ��۲쵽��������___________����Һ����ɫ�����ɫ��
��3���Ѽס��ҷ�Ӧ�����Һ����ͬһ�ջ��������а�ɫ�������ɡ���������ɫ�����ijɷֽ���̽����
���������ϡ����ᱵ�������ᡣ
��������롿 ��ɫ����Ϊ����._______����.Mg(OH)2��BaSO4������Mg(OH)2��MgCO3.
��ʵ�鷽����
ʵ����� | ʵ����������� |
���ˡ�ϴ�Ӱ�ɫ�������ã����ɫ�����м������������� | ��������ȫ���ܽ⣬�����������������������仯ѧ��Ӧ����ʽΪ____________�� ����______������������������������� ����������ȫ�ܽ⣬________�������������� |
����չ��˼������������������������˽����ԭ��������ʵ��ʱ___________��������ɫ�����ijɷֲ����ܳ��ֵ������Mg(OH)2��MgCO3��BaSO4��ԭ����__________________��