��Ŀ����
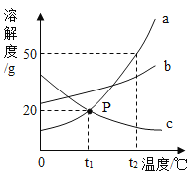
��֪ KNO3���ܽ�����±���ʾ������˵������ȷ����
�¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 | 70 |
�ܽ��/g | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | 138 |
A.�� 10��-70��֮�䣬�����¶ȵ����ߣ�KNO3���ܽ������
B.20��ʱ���� 100 g ˮ�м��� 35 g KNO3����ֽ��裬������Һ����Ϊ 131.6 g
C.�� 70��ʱ�����͵� KNO3��Һ�����¶ȣ��п���ת��Ϊ������Һ
D.50��ʱ������ KNO3��Һ�����ʵ���������Ϊ 85.5%
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���ú�ң���Ҫ����SiO2��Fe2O3��Al2O3�ȣ���ȼú��������в����ķ�������ú�ҵ��ۺ����þ��кܴ�ļ�ֵ��
��1����������Һ�ֽ��ú�ң�ʹ���е�������Ԫ���ܳ�������ʵ�ֳ������롣
��д��������Һ��Fe2O3��Ӧ�ķ���ʽ_______��
�ڳ�������õ���������Ҫ�ɷ���_____��
��2����Ҫ����1�� �������õ���Һ�е�Fe3+ת��ΪFe2+������±�������ԭ��_________��
���� | Fe (OH)2 | Fe (OH)3 | Al (OH)3 |
��������(pH) | 7.06~ 8.95 | 1.94~3.20 | 3.69~4.8 |
��3��ʹ�ü��Խ����İ�ˮΪ pH ���ڼ������з���ʵ�顣
�ٰ�ˮʹ��Һ�е�Al3+�����ĵĻ�ѧ����ʽΪ______��
�ڷ�Ӧ�յ�� pH ֵ������������Ч����Ӱ����ͼ��
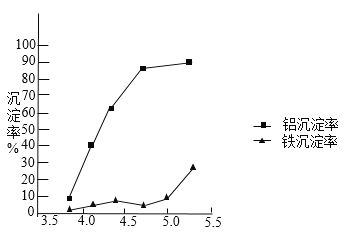
����ʵ������Ϊ�ﵽ�õķ���Ч������Ӧ�����п��� pH ֵԼΪ_____��ѡ��� pHֵ��������_________��



 �㵹Һ�� B.
�㵹Һ�� B.  ��ȡҺ��
��ȡҺ��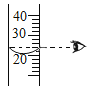 ��ȡҺ�� D.
��ȡҺ�� D. ����Һ��
����Һ��