��Ŀ����
�й�2010���Ϻ��������ڼ䣬�������ڵ�һЩ������������ˡ����У�����������á������⡣
�� �á�����̿+����Ĥ+�����ߡ���Ϲ��ջ��ֱ��ˮ�����л���̿�������� ��
�� ����������ʳ�õ�������ʳ���У����������ʵ��� �������,��ͬ����

�� ʹ�õĵ綯�۹����Ч������CO2��SO2��CO��������ŷţ���Щ����������������ЧӦ���� �������������� ��
�� ʹ�õķ��С��ؿ���֤���ȶ��ÿ���ȫ����ġ��������ϡ��Ƴɣ�����ʱ�������͵õ�����(C3H6O3)�������� ��Ԫ����ɣ�����̼Ԫ�ص���������Ϊ ��
��������ľٰ죬�����Ǹ��ӹ�ע���������������У����ϡ���̼���������� ��
�� �á�����̿+����Ĥ+�����ߡ���Ϲ��ջ��ֱ��ˮ�����л���̿�������� ��
�� ����������ʳ�õ�������ʳ���У����������ʵ��� �������,��ͬ����

�� ʹ�õĵ綯�۹����Ч������CO2��SO2��CO��������ŷţ���Щ����������������ЧӦ���� �������������� ��
�� ʹ�õķ��С��ؿ���֤���ȶ��ÿ���ȫ����ġ��������ϡ��Ƴɣ�����ʱ�������͵õ�����(C3H6O3)�������� ��Ԫ����ɣ�����̼Ԫ�ص���������Ϊ ��
��������ľٰ죬�����Ǹ��ӹ�ע���������������У����ϡ���̼���������� ��
| A����Լʹ����Ȼ�� | B���ᳫʹ��һ����ľ�� |
| C�����ദ���������� | D������ѡ�ù�����ͨ���ٿ��� |
�� ���� �� C �� CO2 SO2 �� 3 40% �� ACD
��������1���ھ���ˮ�Ĺ����У����û���̿����ˮ�е���ɫ����ζ����ˮ��ͨ�����������������߿ɶ�ˮ����ɱ��������
��2������ʳ����Ӫ����֮��Ĺ�ϵ���
��3�����ݻ�����Ⱦ����
��4�����ݵ�̼��������ĺ�����
��𣺽⣺��1����ˮ�����У�����̿���������ã�����ˮ�е���ɫ����ζ��
��2�����Ѹ���ά���أ����������֬�������и��������ʣ�
��3�����������У�����������ЧӦ����CO2��������������SO2��
��4�����Ậ��C��H��O����Ԫ�أ����У�̼Ԫ�ص���������=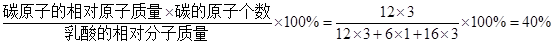 ��5����̼�����������ָ������Ϣʱ�����õ�����Ҫ�������٣��Ӷ�����̼���ر��Ƕ�����̼���ŷ������Ӷ����ٶԴ�������Ⱦ��������̬����Ҫ�Ǵӽڵ�����ͻ��������������ı�����ϸ�ڣ��ʴ�Ϊ����1��������2��C����3��CO2��SO2����4��3��40%����5��ACD��
��5����̼�����������ָ������Ϣʱ�����õ�����Ҫ�������٣��Ӷ�����̼���ر��Ƕ�����̼���ŷ������Ӷ����ٶԴ�������Ⱦ��������̬����Ҫ�Ǵӽڵ�����ͻ��������������ı�����ϸ�ڣ��ʴ�Ϊ����1��������2��C����3��CO2��SO2����4��3��40%����5��ACD��
��2������ʳ����Ӫ����֮��Ĺ�ϵ���
��3�����ݻ�����Ⱦ����
��4�����ݵ�̼��������ĺ�����
��𣺽⣺��1����ˮ�����У�����̿���������ã�����ˮ�е���ɫ����ζ��
��2�����Ѹ���ά���أ����������֬�������и��������ʣ�
��3�����������У�����������ЧӦ����CO2��������������SO2��
��4�����Ậ��C��H��O����Ԫ�أ����У�̼Ԫ�ص���������=
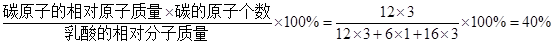 ��5����̼�����������ָ������Ϣʱ�����õ�����Ҫ�������٣��Ӷ�����̼���ر��Ƕ�����̼���ŷ������Ӷ����ٶԴ�������Ⱦ��������̬����Ҫ�Ǵӽڵ�����ͻ��������������ı�����ϸ�ڣ��ʴ�Ϊ����1��������2��C����3��CO2��SO2����4��3��40%����5��ACD��
��5����̼�����������ָ������Ϣʱ�����õ�����Ҫ�������٣��Ӷ�����̼���ر��Ƕ�����̼���ŷ������Ӷ����ٶԴ�������Ⱦ��������̬����Ҫ�Ǵӽڵ�����ͻ��������������ı�����ϸ�ڣ��ʴ�Ϊ����1��������2��C����3��CO2��SO2����4��3��40%����5��ACD��
��ϰ��ϵ�д�
 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
�����Ŀ
