��Ŀ����
 ����������������Ӧ�ù㷺��
����������������Ӧ�ù㷺��
��1���������Ƴ��������������ý������������õ�________�ԣ�
��2������Ʒ�����������________����ϡ��������ⷴӦ�Ļ�ѧ����ʽ��________��
��3���ҹ����������������ʪ��ұ����ȡͭ�Ĺ��ң���������ͭ��Һ��Ӧ�Ļ�ѧ����ʽ��________����ҵ��ұ�������ǽ�����ʯ����Ҫ�ɷ���Al2O3��������������ͨ��ֽ⣬�䷴Ӧ�Ļ�ѧ����ʽ��________��
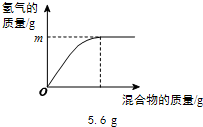
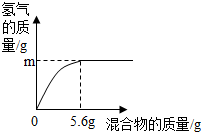
��4���������ֽ���������ɵĻ�����100gijϡ�����м���û�������������������������������ϵ��ͼ��ʾ��
����˵����ȷ����________������ţ���
a���������ΪZn��Al����m������0.2g
b���������ΪZn��Cu����mһ������0.2g
c���������ΪFe��Al����ϡ������������������һ������7.3%
d���������ΪFe��Cu��mΪ0.1g����û������Fe����������һ����50%
�⣺��1���������������õ���չ�ԣ�����������Ƴ�������
��2������Ʒ������������볱ʪ�Ŀ����Ӵ����������Ҫ�ɷ�����������ϡ�������������Ӧ�����Ȼ�����ˮ�������ϡ��������ⷴӦ�Ļ�ѧ����ʽ��Fe2O3+6HCl=2FeCl3+3H2O��
��3����������ͭ��Һ��Ӧ��������������ͭ����Ӧ�Ļ�ѧ����ʽ��Fe+CuSO4=FeSO4+Cu������ʯ����Ҫ�ɷ���Al2O3��������������ͨ��ֽ����������������䷴Ӧ�Ļ�ѧ����ʽ��2Al2O3 4Al+3O2����
4Al+3O2����
��4���������ȫ����������������Ϊ5.6 g������ݻ�ѧ��Ӧ�ķ���ʽ��Fe+2HCl=FeCl2+H2���ɼ������ʱ������������������0.2 g��ͬ���ɼ����5.6 gAl�����ᷴӦ������������������0.2 g��5.6gп�����ᷴӦ��������������С��0.2 g��ͭ�������Ӧ��
a���������ΪZn��Al��m���ܵ���0.2g����a��ȷ��
b���������ΪZn��Cu����mһ��С��0.2g����b����
c���������ΪFe��Alʱ������ͬ������������ϡ����������������������������5.6 g��ʱ���������������Ϊ7.3 g�����Ի����ΪFe��Alʱ�������������������7.3 g����������������ʽ��֪��������������������һ������7.3%����c��ȷ��
d���������ΪFe��Cu��mΪ0.1g������0.1g������Ҫ����������2.8g����������������������Ϊ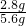 ��100%=50%����d��ȷ��
��100%=50%����d��ȷ��
�ʴ�Ϊ����1����չ����2���볱ʪ�Ŀ����Ӵ���Fe2O3+6HCl=2FeCl3+3H2O����3��Fe+CuSO4=FeSO4+Cu��2Al2O3 4Al+3O2������4��acd��
4Al+3O2������4��acd��
��������1���������ʵ����ʾ�������;������
��2���������������������ϡ��������ԭ�����з�����
��3�����ݷ�Ӧԭ����ϻ�ѧ����ʽ����д������
��4����Ϊ�������ԭ������������п��С��������ͬ����������������ȫ��Ӧʱ�����������������࣮
��������ͬ�����Ľ������ᷴӦ���������������Ķ�������������ԭ�������йأ������ԭ������Խ��������������ԽС��
��2������Ʒ������������볱ʪ�Ŀ����Ӵ����������Ҫ�ɷ�����������ϡ�������������Ӧ�����Ȼ�����ˮ�������ϡ��������ⷴӦ�Ļ�ѧ����ʽ��Fe2O3+6HCl=2FeCl3+3H2O��
��3����������ͭ��Һ��Ӧ��������������ͭ����Ӧ�Ļ�ѧ����ʽ��Fe+CuSO4=FeSO4+Cu������ʯ����Ҫ�ɷ���Al2O3��������������ͨ��ֽ����������������䷴Ӧ�Ļ�ѧ����ʽ��2Al2O3
 4Al+3O2����
4Al+3O2������4���������ȫ����������������Ϊ5.6 g������ݻ�ѧ��Ӧ�ķ���ʽ��Fe+2HCl=FeCl2+H2���ɼ������ʱ������������������0.2 g��ͬ���ɼ����5.6 gAl�����ᷴӦ������������������0.2 g��5.6gп�����ᷴӦ��������������С��0.2 g��ͭ�������Ӧ��
a���������ΪZn��Al��m���ܵ���0.2g����a��ȷ��
b���������ΪZn��Cu����mһ��С��0.2g����b����
c���������ΪFe��Alʱ������ͬ������������ϡ����������������������������5.6 g��ʱ���������������Ϊ7.3 g�����Ի����ΪFe��Alʱ�������������������7.3 g����������������ʽ��֪��������������������һ������7.3%����c��ȷ��
d���������ΪFe��Cu��mΪ0.1g������0.1g������Ҫ����������2.8g����������������������Ϊ
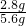 ��100%=50%����d��ȷ��
��100%=50%����d��ȷ���ʴ�Ϊ����1����չ����2���볱ʪ�Ŀ����Ӵ���Fe2O3+6HCl=2FeCl3+3H2O����3��Fe+CuSO4=FeSO4+Cu��2Al2O3
 4Al+3O2������4��acd��
4Al+3O2������4��acd����������1���������ʵ����ʾ�������;������
��2���������������������ϡ��������ԭ�����з�����
��3�����ݷ�Ӧԭ����ϻ�ѧ����ʽ����д������
��4����Ϊ�������ԭ������������п��С��������ͬ����������������ȫ��Ӧʱ�����������������࣮
��������ͬ�����Ľ������ᷴӦ���������������Ķ�������������ԭ�������йأ������ԭ������Խ��������������ԽС��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ


 ��2013?������һģ������������������Ӧ�ù㷺��
��2013?������һģ������������������Ӧ�ù㷺��