��Ŀ����
��9�֣�ȼ�շ���һ�ֳ�����ȷ���л���Ԫ����ɵ�ʵ�鷽����������������⣺
������ʵ�顿
��1������Ļ�ѧʽ��
��2����д��ͨ������ȼ��ȷ�������Ԫ�ص�ʵ�鷽����
������ʵ�顿
ij�л�ʳƷ���ʴ�������̼Ԫ�غ���Ԫ���⣬���ܺ�����Ԫ�ء�Ϊȷ���ñ��ʴ��Ƿ�����Ԫ�أ���������ʵ��̽����
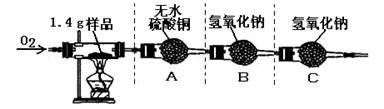
ʵ�����
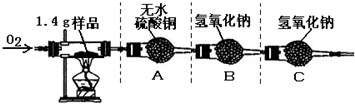
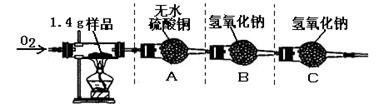
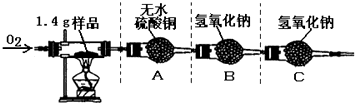
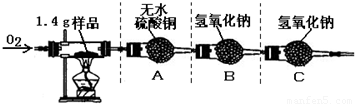
�ٰ�ͼʾ���Ӻ������������װ��������
�ڳ�ȡ1.4 g�ñ��ʴ���Ʒ����������Ӳ�ʲ����Թ��С�
������ƽ�Ƶ�װ��A������Ϊ82.0 g��װ��B������Ϊ85.0 g
�ܳ���ͨ�봿O2����ȼ�ƾ��ƣ�ֱ�����ʴ���Ʒ��ȫȼ��
��ʵ�����������ȴ���ٴ�����ƽ�Ƶ�װ��A������Ϊ83.8g, װ��B������Ϊ89.4g
��ش�
��1��������ϣ���ɫ����ˮ����ͭ����ˮ����ˮ�����ɫ������ʵ���װ��A�й������������˵���ñ��ʴ����� Ԫ�ء�
��2�����������غ㶨�ɣ�װ��B�ڷ�Ӧ�����ӵ���������1.4 g��Ʒ��ȫȼ�պ����ɵ�_____ ���ѧʽ����������
��3��ͨ������ɵã���Ʒ��̼Ԫ�ص�����Ϊ ����Ԫ�ص�����Ϊ ��
��4�����ۣ��ñ��ʴ���Ʒ ������С������С�����Ԫ�ء�
��5������װ��C����ʵ������Ʒ��̼Ԫ�ص������� ���ƫ��ƫС������Ӱ�족��
������ʵ�顿(1)CH4(2) �������ȼ���ڻ����Ϸ���һ�����������ձ���һ������ձ�����ˮ����֣�֤�����麬����Ԫ�أ�Ѹ�ٰ��ձ������������ձ���ע����������ʯ��ˮ�������ֱ���ǣ�֤�������к���̼Ԫ�ء�(������������)����֤��ÿ��Ԫ�ص�1�֣�
������ʵ�顿
(1)�⣨��H�� (2) CO2 (3)1.2 g ��0.2 g
(4) ������ (5)ƫ��
����:������ʵ�顿��1������������л���仯ѧ��Ϊ��CH4
��2�������к���̼Ԫ�غ���Ԫ�أ�ͨ��ȼ�գ���̼Ԫ��ת��Ϊ������̼����Ԫ��ת����ˮ���ʴ�Ϊ���������ȼ���ڻ����Ϸ���һ�����������ձ���һ������ձ�����ˮ����֣�֤�����麬����Ԫ�أ�Ѹ�ٰ��ձ������������ձ���ע����������ʯ��ˮ�������ֱ���ǣ�֤�������к���̼Ԫ�ء�
������ʵ�顿��1����֪��ɫ����ˮ����ͭ����ˮ����ˮ�����ɫ��A�й��������˵����Ӧ��ˮ���ɣ��Ӷ�˵����Ʒ�к�����Ԫ�أ���2�����������غ㶨�ɣ�װ��B�ڷ�Ӧ�����ӵ���������1.4 g��Ʒ��ȫȼ�պ����ɵĶ�����̼����������3������ʵ�����й����ݵã�ʵ�����ɶ�����̼��ˮ�������ֱ�Ϊ��89.4g��85.0g=4.4g;83.8g��82.0g=1.8g;���������غ㶨�ɣ���Ʒ��̼Ԫ�ص�����Ϊ��12�£�12+16��2����4.4g=1.2g;��Ԫ�ص�����Ϊ��2�£�2+16����1.8g=0.2g;(4)��0.2g+1.2g=1.4g�����Զ϶���Ʒ��û����Ԫ�أ���5����װ��C�����������տ����еĶ�����̼�������ӣ���ʹ�������ƫ��

 ��У����ϵ�д�
��У����ϵ�д�