��Ŀ����
��2011?������С�������ʦ����KNO3�ı�����Һ���Ա�ȫ��ʵ��ʹ�ã���ʵ�����¶�Ϊ25�棬��ʦ�ṩ��ҩƷ�Ǻ�������NaCl��KNO3���壮
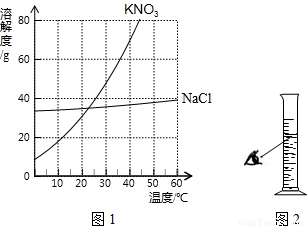
��1�����������������ʵ��ܽ�����ߣ���ͼ1��ʾ��25��ʱ��KNO3���ܽ����

��2��С������700g KNO3������Һ�Ĺ������£�
�ټ��㣻
�ڳ�����ȷ����KNO3����
����ȡ����
���ܽ⣺��KNO3��ˮ�ֱ������ձ��У�����ʹ������ȫ�ܽ⣮
��ָ��С����������Һ�����еIJ�������
���С����õı�����Һװƿ�����ϱ�ǩ���ã�
��1�����������������ʵ��ܽ�����ߣ���ͼ1��ʾ��25��ʱ��KNO3���ܽ����
40
40
g��������KNO3�л�������NaCl���ᴿ��������ȴ�ȱ�����Һ�����½ᾧ��
��ȴ�ȱ�����Һ�����½ᾧ��
���ô˷������������KNO3���壮
��2��С������700g KNO3������Һ�Ĺ������£�
�ټ��㣻
�ڳ�����ȷ����KNO3����
200
200
g������ȡ����
500ml
500ml
���100mL������500mL����1000mL������Ͳ��ȡ����ˮ��������ͼ2��ʾ�����ܽ⣺��KNO3��ˮ�ֱ������ձ��У�����ʹ������ȫ�ܽ⣮
��ָ��С����������Һ�����еIJ�������
���Ӷ�ȡˮ����������������������ɣ�
���Ӷ�ȡˮ����������������������ɣ�
���������ϴ������������õIJ���KNO3������Һ��Ҫ�õ����¶��µı�����Һ�������ܲ��õķ���������ˮ���ܼ���
����ˮ���ܼ���
�����С����õı�����Һװƿ�����ϱ�ǩ���ã�
���������ܽ�����ߣ����Բ��25��ʱ��KNO3���ܽ�ȣ���KNO3���ܽ�����¶ȵ�Ӱ��仯���ƣ�����ȷ���ᾧ�����ķ������ɱ�����Һ�����ʡ���Һ��������ϵ���Լ�����������ʡ��ܼ���������С����������Һ�����еIJ��������Ӷ�ȡˮ��������ᵼ����ȡˮ�����ƫ����õIJ���KNO3������Һ��Ҫ�õ����¶��µı�����Һ�������Բ�������ˮ�ķ�����
����⣺��1����ͼ1��֪��25��ʱ��KNO3���ܽ����40g��KNO3���ܽ�����¶ȵ�Ӱ��ϴʿɲ�����ȴ�ȱ�����Һ�����½ᾧ���ķ����ᴿ��
��2����������KNO3���������Ϊx����40g��140g=x��700g��x=200g������ˮ������Ϊ��700g-200g=500g������Ҫ500ml����Ͳ��ȡˮ��
��С����������Һ�����еIJ��������Ӷ�ȡˮ��������ᵼ����ȡˮ�����ƫ����õIJ���KNO3������Һ��Ҫ�õ����¶��µı�����Һ�������Բ�������ˮ�ķ�����
�ʴ�Ϊ��
��1��40����ȴ�ȱ�����Һ�����½ᾧ��
��2����200��500mL�����Ӷ�ȡˮ����������������������ɣ�������ˮ���ܼ���
��2����������KNO3���������Ϊx����40g��140g=x��700g��x=200g������ˮ������Ϊ��700g-200g=500g������Ҫ500ml����Ͳ��ȡˮ��
��С����������Һ�����еIJ��������Ӷ�ȡˮ��������ᵼ����ȡˮ�����ƫ����õIJ���KNO3������Һ��Ҫ�õ����¶��µı�����Һ�������Բ�������ˮ�ķ�����
�ʴ�Ϊ��
��1��40����ȴ�ȱ�����Һ�����½ᾧ��
��2����200��500mL�����Ӷ�ȡˮ����������������������ɣ�������ˮ���ܼ���
�����������ѶȲ�����Ҫ�����˹����ܽ����������ʾ�����塢һ����������������Һ�����ƣ�ͨ��������Լ�ǿѧ���Թ����ܽ�ȵ����⣬����ѧ��Ӧ��֪ʶ��������������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��2011?������С���ڼ�ϴ�·�ʱ������һƿ�չ��ڵ�Ư��Һ����Ư��Һ��Ư��ԭ����Ư��Һ�Ƿ���Ư�����ò��������ʣ����ǽ������ѧУ������ʦ��ָ���£���С��ͬѧһ��չ��̽����
���������ϡ�
����ȡƯ��Һ��ԭ����Cl2+2NaOH�TNaClO+NaCl+H2O������Ч�ɷ���NaClO��
��Ư��Һ��Ư��ԭ����
NaClO�ڿ����кܿ췢����Ӧ��2NaClO+H2O+CO2�TNa2CO3+2HClO
���ɵ�HClO��ʹ��ɫ���������л�ɫ�أ���ɫ��
��HClO���ȶ����ֽ⣬�ֽ��ɥʧƯ�����ã�
��������⡿�չ��ڵ�Ư��Һ�Ƿ�ʧЧ��
��ʵ��̽������С���ʵ�鱨�����£�
С��ͬѧ��ʧЧ��Ư��Һ����Ҫ�ɷֺܸ���Ȥ����������벢����һ��̽����
��������롿С�������У�NaCL
СӢ�����У�NaCl��Na2CO3
�������NaCl��Na2CO3��NaOH
����Ʒ�����
С��ͬѧ�������ۣ���Ϊ��������ϡ����Ϳ�����֤______�IJ��������
Ϊ��֤����λͬѧ�IJ��룬������������·�����
��������ۺ�С��ͬѧ����ƣ���ʵ��ó��˽��ۣ�
���������ϡ�
����ȡƯ��Һ��ԭ����Cl2+2NaOH�TNaClO+NaCl+H2O������Ч�ɷ���NaClO��
��Ư��Һ��Ư��ԭ����
NaClO�ڿ����кܿ췢����Ӧ��2NaClO+H2O+CO2�TNa2CO3+2HClO
���ɵ�HClO��ʹ��ɫ���������л�ɫ�أ���ɫ��
��HClO���ȶ����ֽ⣬�ֽ��ɥʧƯ�����ã�
��������⡿�չ��ڵ�Ư��Һ�Ƿ�ʧЧ��
��ʵ��̽������С���ʵ�鱨�����£�
| ʵ����� | ʵ������ | ʵ����� |
| ȡ������Ư��Һ���ձ��У�______ | ______ | ��Ư��Һ����ȫʧЧ |
��������롿С�������У�NaCL
СӢ�����У�NaCl��Na2CO3
�������NaCl��Na2CO3��NaOH
����Ʒ�����
С��ͬѧ�������ۣ���Ϊ��������ϡ����Ϳ�����֤______�IJ��������
Ϊ��֤����λͬѧ�IJ��룬������������·�����
| ʵ�鲽�� | Ԥ��ʵ������ | ʵ��Ŀ�Ļ�Ԥ�ڽ��� |
| ����٣�ȡ������Ư��Һ���Թ��У�����______�����ã��۲� | ������ɫ���� | Ŀ�ģ� ______ |
| ����ڣ�ȡ�ϲ���Һ���Թ��У�______���۲� | ______ | ���ۣ� ______���������������һλͬѧ��������� |