��Ŀ����
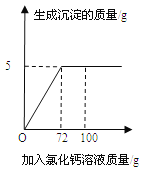
����Ŀ�����ձ���100.0gϡ�����м���һ����ͭп�Ͻ��ĩ���Ȳ���������ð�����õ������ձ���ͨ�����������ȣ���ĩ���١���ʧ����Һ����ɫ��Ϊ��ɫ�������Һ������ʵ��ʱ��Ĺ�ϵ��ͼ��

����˵��������ǣ�������
A.��a�㵽b�㣬����0.4g H2
B.��b�㵽c�㣬˵��Cu�����û��������е���
C.��c�㵽d�㣬�����ķ�ӦΪ2Cu+2H2SO4+O2 ![]() 2CuSO4+2H2O
2CuSO4+2H2O
D.��d�㵽e�㣬��Һ�е����������ж���
���𰸡�A
��������
���ձ���100.0gϡ�����м���һ����ͭп�Ͻ��ĩ��п������ϡ���ᷴӦ���Ȳ���������ð�����õ������ձ���ͨ�����������ȣ���ĩ���١���ʧ����Һ����ɫ��Ϊ��ɫ��ͭ��������ϡ���ᷴӦ��������ͭ��ˮ��
�⣺A����a�㵽b�㣬��Һ��������6.3g������
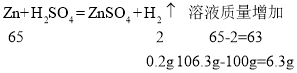 ͨ����ѧ����ʽ��֪������0.2g������˵�����������⣻
ͨ����ѧ����ʽ��֪������0.2g������˵�����������⣻
B��ͭ�Ľ������������ĺ��棬���Դ�b�㵽c����Һ�������䣬˵��Cu�����û��������е��⣬˵����ȷ�����������⣻
C����c�㵽d�㣬�����ķ�Ӧͭ��������ϡ���ᷴӦ��������ͭ��ˮ����ѧ����ʽΪ��2Cu+2H2SO4+O2 ![]() 2CuSO4+2H2O��˵����ȷ�����������⣻
2CuSO4+2H2O��˵����ȷ�����������⣻
D����d�㵽e�㣬��Һ�е�����һ����������п������ͭ���֣����ܺ���ϡ���ᣬ˵����ȷ�����������⡣��ѡA��

����Ŀ����Ũ����Ĵ������£�������ᣨH2C2O4�����ȷֽ�����̼���������ˮ��ij��ѧ������ȤС�����������̼���������������������ʵ��̽����
��������⣩��������������̼�������
���²⣩
����1 | ����2 | ����3 |
ֻ��CO | ֻ��CO2 | ����CO��CO2 |
�����ʵ�飩���ڲ���3������CO��CO2�����ʣ���ȤС��ͬѧ���������ʵ�飺

(1)�۲쵽_____������ĸ�� װ���еij���ʯ��ˮ����ǣ�֤������ֽ���CO2�������ɡ�
(2)������ʵ�������ֱܷ�֤������ֽ�������к���CO����Cװ���г���ʯ��ˮ������ǣ�Fװ����_________����Eװ���г���________������
��ʵ����ۣ�ͨ��ʵ��̽��֤��������3����������ֽ�Ļ�ѧ����ʽ��____________________��
���������ۣ���װ��B������������Һ��������____________��
��װ��ĩ�˾ƾ��Ƶ�������________________��