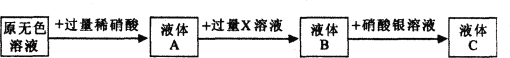��Ŀ����
����Ŀ��(5��)ijˮ�¹����豸�й������ǹ�������(Na2O2)��������������ˮ��Ӧ��������������Ӧ�Ļ�ѧ����ʽΪ��2Na2O2 + 2H2O == 4NaOH + O2��
ijͬѧΪ��һ��̽������������ˮ�ķ�Ӧ�и�����������ϵ����15��6g�������Ƽ���ʢ��147��6gˮ(����)���ձ��г�ַ�Ӧ��ֱ��������ȫ��ʧ�����������ݲ���������㣺
��1����Ӧ���ɵ�����������
��2����Ӧ��������Һ�����ʵ�����������
���𰸡���1��3.2g ��2��10%
��������
�������������������������Ϊx�������������Ƶ�����Ϊy
2Na2O2 + 2H2O == 4NaOH + O2��
156 160 32
15.6g y x
156/15.6g=32/x x=3.2g
156/15.6g=160/y y=16g
NaOH ����������==![]() ==10%
==10%
����1����������3.2g
��2����Ӧ��������Һ�����ʵ���������Ϊ10%

��ϰ��ϵ�д�
�����Ŀ