��Ŀ����
����Ŀ��ijʵ��С������ͼ��ʾװ�ò����������������������ȡ�óɹ���
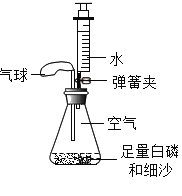
[��������]�����Ż��40�森
[�������]�������Լռ����������Ķ��٣�
[ʵ����]��ƿ�ڿ������Ϊ230mL��ע������ˮ�����Ϊ50mL����װ�����������ã�
[ʵ��̽��]װ��ҩƷ����ͼ��ʾ���Ӻ��������н����ɼУ��Ƚ���ƿ�ײ�������ˮ�У����ܿ챻��ȼ��Ȼ����ƿ����ˮ��ȡ����
[ʵ�����]
��1������ƿ�ײ�������ˮ�У����ױ���ȼ��˵��ȼ�ղ���ȱ�ٵ�һ��������_____�������İ�������ƿ��δ��ȫ��ȼ�գ�˵��ƿ��ʣ������_____ȼ�գ�����֧����������֧��������д������ȼ�յ����ֱ���ʽ��_____��
��2��������ʵ������У��ɹ۲쵽����ı仯��_____��
��3��������Ϩ����ƿ��ȴ�����º��ɼУ����ɹ۲쵽�������ǣ���ע�����е�ˮ�Զ�����������ڵ�ע�����е�ˮ��ʣԼ4mLʱֹͣ������������Щ��������ԭ���ǣ�_____��_____��
��4������ƿ�ڿ�������IJ���������_____��
��5��ͨ�������ʵ�飬��ѧ���IJ����������ij�ɷֺ����ķ�����_____��
���𰸡��¶ȴﵽ�Ż�� ��֧�� ��+����![]() ���������� �ȱ����С ����ȼ����������ƿ�е�������ʹƿ����ѹ��С ��ƿ������Լռ46mL����ע�����е�ˮ������ƿ46mL��ƿ����ѹ������൱ �Ƚ���ƿע��ˮ������Ͳ����ˮ��������ɵõ���ƿ�ڿ������ ͨ����ѧ��Ӧ��ȥ������е�һ�ֳɷ֣��ٲ���������ڷ�Ӧǰ����������������ı仯���Ӷ��ó����ֳɷֵĺ���
���������� �ȱ����С ����ȼ����������ƿ�е�������ʹƿ����ѹ��С ��ƿ������Լռ46mL����ע�����е�ˮ������ƿ46mL��ƿ����ѹ������൱ �Ƚ���ƿע��ˮ������Ͳ����ˮ��������ɵõ���ƿ�ڿ������ ͨ����ѧ��Ӧ��ȥ������е�һ�ֳɷ֣��ٲ���������ڷ�Ӧǰ����������������ı仯���Ӷ��ó����ֳɷֵĺ���
��������
��1������ƿ�ײ�������ˮ�У����ܿ챻��ȼ��˵��ȼ�ղ���ȱ�ٵ�һ�������ǿ�ȼ����¶ȴﵽ�Ż�㣮����ȼ�����������������ף���Ӧ�����ֱ���ʽΪ����+����![]() ���������ף�ƿ��ʣ�������ǵ�������֧��ȼ�գ�
���������ף�ƿ��ʣ�������ǵ�������֧��ȼ�գ�
��2������ȼ��ʱ�ų��������ȣ�ʹƿ��ѹǿ�������ڰ���ȼ������������������ȴ�����£�ƿ��ѹǿ��С�����Կɹ۲쵽����ı仯�ǣ��ȱ����С��
��3������ȼ����������ƿ�е�������ʹƿ����ѹ��С��ע�����е�ˮ�Զ����������ƿ������Լռ46mL����ע�����е�ˮ������ƿ46mL��ƿ����ѹ������൱�����ԣ���ע�����е�ˮ��ʣ��Լ4mLʱֹͣ������
��4������ƿ�ڿ�������IJ����������Ƚ���ƿע��ˮ������Ͳ����ˮ��������ɵõ���ƿ�ڿ��������
��5����������ʵ���֪�������������ij�ɷֺ����ķ�����ͨ����ѧ��Ӧ��ȥ������е�һ�ֳɷ֣��ٲ���������ڷ�Ӧǰ����������������ı仯���Ӷ��ó����ֳɷֵĺ�����

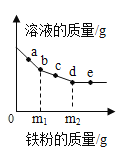
����Ŀ���±��г���20��ʱNaCl�ܽ�ʵ���һ������
ʵ����� | ˮ������/g | ����NaCl������/g | ������Һ������/g |
�� | 10 | 2 | 12 |
�� | 10 | 3 | 13 |
�� | 10 | 4 | 13.6 |
�� | 10 | 5 | 13.6 |
����������ȷ����
A���٢�������Һ��20��ʱNaCl�IJ�������Һ
B���٢ڢ�������Һ��20��ʱNaCl�IJ�������Һ
C��20��ʱNaCl���ܽ��Ϊ3.6g
D����������Һ�����ʵ���������Ϊ20%
����Ŀ����1���ҹ��������й�ˮ�ܡ������������Ϊ����ʵ���չ����ƽ����ϸ�ˮ��Դ��������ˮ������֮Դ��ˮ����������������������أ���ش��������⣺
��Ҫ�������ǵ��ص�����ˮ��Ӳˮ������ˮ������Ϊ������_____������
�ڵ��ˮʱ�����븺���������в��������������ԼΪ_____��
��2��ʵ���ҳ��ü����������������̻����ķ�����ȡ������д����Ӧ�����ֱ���ʽ��_____
С�����֣������������ͭ��ϼ��ȣ�Ҳ�ܽϿ�������������ǽ�������̽����
��������룩��MnO2��CuO�⣬Fe2O3Ҳ������KClO3�ֽ�Ĵ�����
�����ʵ�飩���±��������飺�ⶨ�ֽ��¶�(�ֽ��¶�Խ�ͣ���Ч��Խ��)��
ʵ���� | ʵ��ҩƷ | �ֽ��¶�(��) |
�� | KClO3 | 580 |
�� | KClO3��MnO2(������1:1) | 350 |
�� | KClO3��CuO(������l:1) | 370 |
�� | KClO3��Fe2O3(������1:1) | 390 |
���������ݡ��ó����ۣ�
����ʵ��_____�γɶԱȣ�֤�����������
��ʵ�����õ����ֽ����������Ч����õ���_____��
����˼��
����Ҫ֤��Fe2O3�Ǹ÷�Ӧ�Ĵ�������Ҫ��֤���ڻ�ѧ��Ӧǰ��������_____��
��ͬ�ִ�����������С����Ӱ���Ч���������ʵ�鷽��������֤_____