��Ŀ����
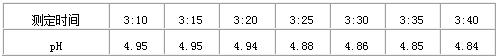
ijУ�����ꡱ��pH��5.6������С���ͬѧ��ȡ�ս�����ˮ��ˮ������pH̽ͷ����pH��������ÿ�������Ӳ�һ��pH�����������£�
| �ⶨʱ�� | 5��05 | 5��10 | 5��15 | 5��20 | 5��25 | 5��30 | 5��35 |
| PH | 4.95 | 4.94 | 4.94 | 4.88 | 4.86 | 4.85 | 4.85 |
��2�������������ݣ��ж�������ˮ�Ƿ�Ϊ�����ꡱ�����ǻ��________��
��3�������飬��һ������һ�����᳧���������̲���SO2����һ����Ƴ�����Щ��ʹ�õ�ȼ����Ҫ��ú���Է��������һ�����������Ҫԭ����________��
��4����ҵ�ϣ�����________��ȥβ���е�SO2����ԭ���û�ѧ����ʽ��ʾΪ________��
�⣺��1��������̼����ˮ��Ӧ����̼�ᣬ̼������ԣ���������к��ж�����̼��������ˮ�������ˮ�����ԣ�
��2���Ǹ���ˮ��pH��5.6���������꣬����ǣ�
��3��ú�к��е���Ԫ�ػ�ת�����ɶ����������᳧�������̲���SO2��������������ˮ��Ӧ���������ᣬ�γ����꣬������᳧�������̲�����SO2�͵�Ƴ�úȼ��Ҳ����SO2����4���������������������Ʒ�Ӧ�����������ƺ�ˮ���Ӷ���ȥ����������������ƣ�2NaOH+SO2=Na2SO3+H2O��
�������������е�֪ʶ���з�������Һ��pHС��7����Һ�����ԣ�������̼����ˮ��Ӧ����̼�ᣬ������ָpH��5.6����ˮ��ú�к��е���Ԫ�ػ�ת�����ɶ������������������������Ʒ�Ӧ�����������ƺ�ˮ��
���������⿼������Һ���������pH�Ĺ�ϵ����ɴ��⣬�����������е�֪ʶ���У�Ҫ��ͬѧ�����������й����ʵ����ʣ��Ա����Ӧ�ã�
��2���Ǹ���ˮ��pH��5.6���������꣬����ǣ�
��3��ú�к��е���Ԫ�ػ�ת�����ɶ����������᳧�������̲���SO2��������������ˮ��Ӧ���������ᣬ�γ����꣬������᳧�������̲�����SO2�͵�Ƴ�úȼ��Ҳ����SO2����4���������������������Ʒ�Ӧ�����������ƺ�ˮ���Ӷ���ȥ����������������ƣ�2NaOH+SO2=Na2SO3+H2O��
�������������е�֪ʶ���з�������Һ��pHС��7����Һ�����ԣ�������̼����ˮ��Ӧ����̼�ᣬ������ָpH��5.6����ˮ��ú�к��е���Ԫ�ػ�ת�����ɶ������������������������Ʒ�Ӧ�����������ƺ�ˮ��
���������⿼������Һ���������pH�Ĺ�ϵ����ɴ��⣬�����������е�֪ʶ���У�Ҫ��ͬѧ�����������й����ʵ����ʣ��Ա����Ӧ�ã�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ