��Ŀ����
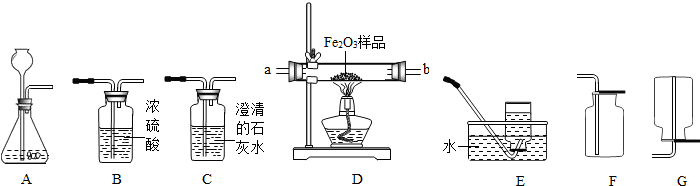
����ʵ��Ҫ����������ͼ��ʾΪʵ���ҳ��õ�ʵ��װ�ã���Ҫ��ش����⣺

��1��д����������������ƣ��� ���� ��
��2��ʵ�������Ը������Ϊԭ����װ��A����ȡ��������װ��A��Ҫ�Ľ��ĵط��� ����Ӧ�Ļ�ѧ����ʽ�� ��
��3��ʵ���ҿ�����ʯ��ʯ��ϡ������ȡCO2�����⣬CO2Ҳ������̼�����ƣ�NaHCO3��������ȷֽ⣨����Ϊ̼���ơ�������̼��ˮ������ȡ���÷�Ӧ�Ļ�ѧ����ʽΪ �����ô˷�����ȡCO2��Ӧѡ�õķ������ռ�װ���� ������ţ���
��4��װ��F�������������ɵ�CO2����������������ˮ���Ϸ�һ��ֲ���͵�Ŀ���� ��ֲ�����Ϸ�ԭ�еĿ�����ʵ��Ľ�� ����С���û�С�������Ӱ�죮
��5�������ü����С��Ͳ�ͷ�ҩƿ��װ��һ����װ�ã���ͼ��ʾ���������ͼ�е�װ��B������ֻ�輫������Һ������Ҳ���٣���ֻ��1��2С�Σ��������װ��B��ʵ����ŵ��� ������ţ���
�ٽ�ԼҩƷ���� ������ȫ������Һ�ŷ�
���ܿ���Һ��ĵμ��ٶ� �ܲ��������岻���κ�����
��6�����ͼ����ȥ�����л��е�����������̼���壬���ռ�һƿ����������ʱ�������������������� ���a����b�����˽���ϴ��ƿ���ø�װ����֤������̼�Ƿ���ˮ��Ӧ���ṩ��ҩƷ�У�
����ɫʯ����Һ������ɫʯ����ҺȾ�ɵĸ���ֽ����
A��B��Ӧ����ʢ�� ����ҩƷ��ţ���

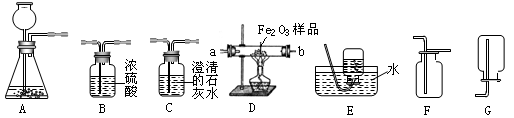
��1��д����������������ƣ���
��2��ʵ�������Ը������Ϊԭ����װ��A����ȡ��������װ��A��Ҫ�Ľ��ĵط���
��3��ʵ���ҿ�����ʯ��ʯ��ϡ������ȡCO2�����⣬CO2Ҳ������̼�����ƣ�NaHCO3��������ȷֽ⣨����Ϊ̼���ơ�������̼��ˮ������ȡ���÷�Ӧ�Ļ�ѧ����ʽΪ
��4��װ��F�������������ɵ�CO2����������������ˮ���Ϸ�һ��ֲ���͵�Ŀ����
��5�������ü����С��Ͳ�ͷ�ҩƿ��װ��һ����װ�ã���ͼ��ʾ���������ͼ�е�װ��B������ֻ�輫������Һ������Ҳ���٣���ֻ��1��2С�Σ��������װ��B��ʵ����ŵ���
�ٽ�ԼҩƷ���� ������ȫ������Һ�ŷ�
���ܿ���Һ��ĵμ��ٶ� �ܲ��������岻���κ�����
��6�����ͼ����ȥ�����л��е�����������̼���壬���ռ�һƿ����������ʱ��������������������
����ɫʯ����Һ������ɫʯ����ҺȾ�ɵĸ���ֽ����
A��B��Ӧ����ʢ��
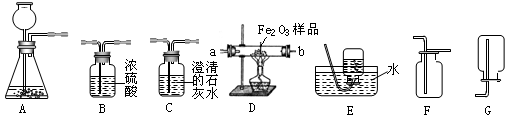
��������1���������ճ����Ļ�ѧ�������Ƽ���;��
��2�������ø��������ȡ������ע�������������д����Ӧ�ķ�Ӧ����ʽ��
��3�����ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ú��ռ�װ�ã�
��4�����ݶ�����̼������ˮ������
��5��������Ϣ�������������ص������
��6������ʱ�������̳�������֤������̼���ų�������̼�����������ԣ�����֤������̼��ˮ��Ӧ�����ԣ�
��2�������ø��������ȡ������ע�������������д����Ӧ�ķ�Ӧ����ʽ��
��3�����ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ú��ռ�װ�ã�
��4�����ݶ�����̼������ˮ������
��5��������Ϣ�������������ص������
��6������ʱ�������̳�������֤������̼���ų�������̼�����������ԣ�����֤������̼��ˮ��Ӧ�����ԣ�
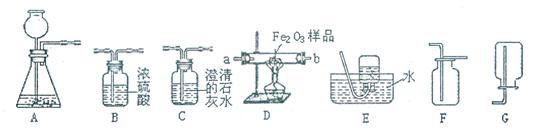
����⣺��1�����dz���©�������Ǽ���ƿ��
��2����Ϊ�������С�����ڼ���ʱ���뵼���ܣ�����Ҫ���Թܿڷ�һ��������Ӧ�ķ���ʽΪ��2KMnO4
K2MnO4+MnO2+O2����
��3��������Ϣд����Ӧ�ķ���ʽΪ��2NaHCO3
Na2CO3+H2 O+CO2��������̼�����ƣ�NaHCO3������ķ���װ�����ڹ�������ͣ���ѡ��ķ���װ��Ϊ��A����Ϊ������̼������ˮ���ܶȱȿ����ʲ��������ſ������ռ���D����
��4����Ϊ������̼������ˮ����ˮ���Ϸ�һ��ֲ���͵�Ŀ���ǣ���ֹ������̼��������ˮ����ˮ��Ӧ��ֲ�����Ϸ�ԭ�еĿ�����ʵ��Ľ��û��Ӱ�죬��Ϊ��ˮ������ȡ�õ�ԭ����
��5������Ϣ������װ�ÿ��Խ�ԼҩƷ����ע�������Կ���Һ������٣���ѡ��٢ۣ�
��6������ʱ����ӳ��ܣ�a������ʹ��Ӧ���ף���֤������̼���ų�������̼�����������ԣ�����֤������̼��ˮ��Ӧ�����ԣ���˳��Ϊ���ڢ�
�ʴ�Ϊ����1���ٳ���©���� �ڼ���ƿ����2���Թ��е��ܿڷ�һ������2KMnO4
K2MnO4+MnO2+O2��
��3��2NaHCO3
Na2CO3+H2 O+CO2����AD����4����ֹ������̼��������ˮ����ˮ��Ӧ��û�С���5���٢ۣ�6��a���ڢ�
��2����Ϊ�������С�����ڼ���ʱ���뵼���ܣ�����Ҫ���Թܿڷ�һ��������Ӧ�ķ���ʽΪ��2KMnO4
| ||
��3��������Ϣд����Ӧ�ķ���ʽΪ��2NaHCO3
| ||
��4����Ϊ������̼������ˮ����ˮ���Ϸ�һ��ֲ���͵�Ŀ���ǣ���ֹ������̼��������ˮ����ˮ��Ӧ��ֲ�����Ϸ�ԭ�еĿ�����ʵ��Ľ��û��Ӱ�죬��Ϊ��ˮ������ȡ�õ�ԭ����
��5������Ϣ������װ�ÿ��Խ�ԼҩƷ����ע�������Կ���Һ������٣���ѡ��٢ۣ�
��6������ʱ����ӳ��ܣ�a������ʹ��Ӧ���ף���֤������̼���ų�������̼�����������ԣ�����֤������̼��ˮ��Ӧ�����ԣ���˳��Ϊ���ڢ�
�ʴ�Ϊ����1���ٳ���©���� �ڼ���ƿ����2���Թ��е��ܿڷ�һ������2KMnO4
| ||
��3��2NaHCO3
| ||
���������⿼���˳����������ȡ�����ʼ���չ֪ʶ������ƽʱ���е�֪ʶ���ɽ����

��ϰ��ϵ�д�
�����Ŀ