��Ŀ����
�Ҵ��׳ƾƾ���������ҽ��������Ҳ����ȼ�ϡ�����ȫȼ�յĻ�ѧ����ʽ�ɱ�ʾΪ��C2H6O+3O2 2 CO2+ 3 H2O ��
2 CO2+ 3 H2O ��
23g�Ҵ���ȫȼ�������Ķ��ٿ�������
�Ҵ�����ȫȼ�ջ����һ����̼��ijʵ���÷�Ӧǰ������ʵ��������±���
| ���� | �Ҵ� | ���� | ������̼ | ˮ | һ����̼ |
| ��Ӧǰ������g�� | 4.6 | 8.8 | 0 | 0 | 0 |
| ��Ӧ��������g�� | 0 | 0 | 6.6 | 5.4 | a |
�ٱ���a��ֵΪ____ __��
�ڸ�ʵ�鷢����Ӧ�Ļ�ѧ����ʽΪ��4C2H6O+11O2
 _____ CO2+ _____ H2O + _____ CO ��
_____ CO2+ _____ H2O + _____ CO ��
(1) 48g
(2) ��1.4 �� 6 12 2
����

��ϰ��ϵ�д�
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
�����Ŀ
��֪20��ʱ����ص��ܽ��Ϊ31.6g���ڸ��¶��½�20g����ط���50gˮ�У���ֽ��裬��������Һ�����ʵ���������ԼΪ
| A��24.0% | B��28.6�� | C��31.6�� | D��40.0�� |
����ݻ�ѧ����ʽ���㣺��ȫ�ֽ�340g������������Ϊ10%�Ĺ���������Һ�еĹ������⣨H2O2����������������������
| A��16g | B��160g | C��32g | D��1.6g |
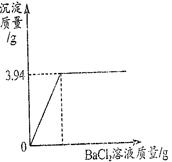
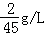 ������
������