��Ŀ����
�������Ѿ����յ�ʵ�����й�������ȡ�;�����֪ʶ�ش��������⣺
��д����ͼ��a�� b�������ƣ�a �� b ��
��ʵ�����ø��������ȡ����ʱ����ѡ�õķ���װ���� ����װ�ô��ţ�������Cװ���ռ����������ȷ�������Ѿ��ռ��� ��ʵ�����ʱ����ȷ����˳��Ϊ���� �� ��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
������Fװ���ռ�O2����O2Ӧ�ӵ��ܿ� ���c����d����ͨ�롣
������Eװ���ռ�ij�����壬���������Ӧ�߱��������� ����д��һ����ȡ��������Ļ�ѧ����ʽ�� ��
��ij����С���ͬѧ����ͼBװ��ʹ����ֽⲢ������װ��̽������ֽ�IJ��


�ٸ�װ���У�A�������� ��C�������� �� A��B (��ܡ����ܡ�)���ཻ����
��ʵ��۲쵽A����ˮ����ͭ������B��F������ʯ��ˮ������ǣ�C������ʯ��ˮ��������E����ɫ��ĩ��ɺ�ɫ��˵������ֽ�IJ����� ��
�� �ƾ��� ˮ��
�� B ƿ��������ð��ʱ֤���������ռ��� �ѵ����Ƴ�ˮ�� Ϩ��ƾ���
2KMnO4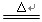 K2MnO4 + MnO2 + O2��
K2MnO4 + MnO2 + O2��
�� c
�� �ܶȱȿ���С Zn + H2SO4 ="=" ZnSO4 + H2�� (��������)
�ɢ� ��������Ƿ���ˮ ����CO2�Ƿ��ѳ��� ����
�� H2O��CO2��CO����:
��
�� B ƿ��������ð��ʱ֤���������ռ��� �ѵ����Ƴ�ˮ�� Ϩ��ƾ���
2KMnO4
 K2MnO4 + MnO2 + O2��
K2MnO4 + MnO2 + O2�� �� c
�� �ܶȱȿ���С Zn + H2SO4 ="=" ZnSO4 + H2�� (��������)
�ɢ� ��������Ƿ���ˮ ����CO2�Ƿ��ѳ��� ����
�� H2O��CO2��CO����:
��

��ϰ��ϵ�д�
 ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
�����Ŀ