��Ŀ����
����Ŀ��ʵ������һƿ���ڱ�¶�ڿ����е��������ƹ��壬ij��ȤС���ͬѧ�Ը���Ʒ�ijɷּ�����������̽����
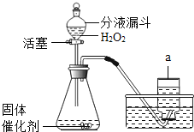
��ʵ��̽��һ��Ϊ�õ��������������ƹ��壬���ⶨNaOH�Ĵ��ȣ���Ƶ�ʵ���������ͼ����ش��������⣺
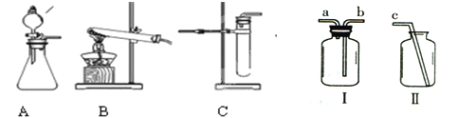
�ٳ��ڱ�¶�ڿ����е��������ƹ�����Ʒ��������������1�� ������B�м�����Լ�����2�� ����������д��ѧʽ���������ƾ�����
�ڲ���A��B �����Ʒֱ�Ϊ��3�� ����4�� ��
����Ҫ�ⶨ�ù�����NaOH�Ĵ��ȣ�ʵ�������һ�������õ�����������5�� ��
��ʵ��̽������Ϊ�˲ⶨNaOH�Ĵ��ȣ�С��ͬѧ�����ͼװ��������̨��ȥ������֪��̼��������Һ�����ն�����̼��
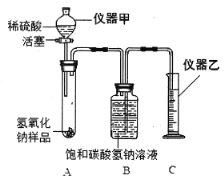
��д��ָ�����������ƣ�
����6�� ������7�� ��
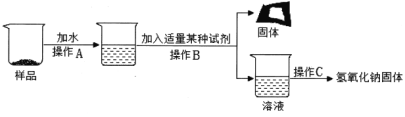
��ʵ����ȡ����Ʒ����Ҫ������һ����Χ�ڣ������Ʒ�������࣬��ɵĺ������8�� ��
��ȡl0g��Ʒ����ʵ�飬����ʵ�����ݣ�ͨ�������֪������CO2 0.11g ����ԭ��Ʒ��NaOH�Ĵ��ȣ�д��������̡�
��9����ʵ�鷴˼��
�����ø�ʵ��װ�ã���������ȷ��װ�����������ã��ⶨ��NaOH����Ҳ����10�� ���ƫ��ƫС��������������11�� ��
���𰸡���1��Na2CO3��
��2��Ca��OH��2
��3���ܽ�
��4������
��5��������ƽ
��6����Һ©��
��7����Ͳ
��8����Ʒ���࣬�кͷ�Ӧ����̫�ർ���������ͣ��ⶨ���ƫ��
��9��97.35%
��10��ƫ��
��11��װ�������ղ����Ķ�����̼���ž�
��������
����������٢ڱ�¶�ڿ����е������������������̼��Ӧ����̼���ƶ����ʣ�ͨ������B��õ��˹����Һ�壬˵������B�ǹ��ˣ�������Լ���̼����ת��Ϊ�˳������������õ������������ƣ���ô������Լ����������ƣ�����������̼���Ʒ�Ӧ����̼��Ƴ������������ƣ������ˮ�ᷢ���ܽ⣬�ʲ���A���ܽ⣻
����Ҫ�ⶨ�ù�����NaOH�Ĵ��ȣ���ôҪ֪����Ʒ�����������ɵij���������������Ҫ������ƽ��
����ͼ��֪����ʵ���м����ҩƷ�����ᣬ����Ȼ���̼���Ʒ�Ӧ���ɶ�����̼Ҳ�����������Ʒ����кͷ�Ӧ�����кͷ�Ӧ�Ƿ��ȷ�Ӧ������Ʒ���࣬��ô�кͷ�Ӧ����̫�ർ���������ͣ��ⶨ���ƫ��
��̼���������ᷴӦ���������ơ�ˮ�Ͷ�����̼�����û�ѧ����ʽ���ݶ�����̼��̼���Ʒ�Ӧ�������ȼ��ɼ����̼���Ƶ���������Ʒ��������ȥ̼���Ƶ�������Ϊ��Ʒ���������Ƶ�������
��̼���Ƶ�����Ϊx��
Na2CO3+H2SO4==Na2SO4+H2O+CO2��
106 44
X 0.11g
106��44 =x��0.11g
X=0.265g
��ô��Ʒ���������Ƶ�����=10g-0.265g=9.735g
�������Ƶ���������=9.735g��10g ��100% =97.35%
������װ�������ղ����Ķ�����̼���ž����ʼ������̼���Ƶ�����ƫС����ô�������Ƶ�����������ƫ��