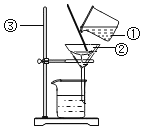
��Ŀ����
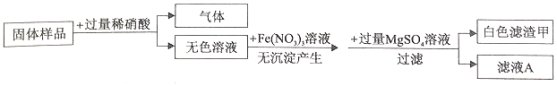
����Ŀ��ij��ɫ������BaSO4��BaCO3��NaCO3��Ba��OH��2�е�����������ɡ�Ϊȷ����ɷ֣�ijͬѧ��Ʋ��������ʵ�飬��ѱ����е����ݲ�������������֪��BaSO4������ϡ���ᣩ

��� | �� | �� | �� |
���� |
|
| �ȼ����̪��Һ�ټ��루3��_____
|
���� | �����ݲ�����������ʣ�� | �����ݲ��� | ������ɫ��������Һ�����ԣ�4��_____ɫ |
���ۻ���� | ԭ��ɫ�����к��У�1��_____ | ԭ��ɫ�����к���Na2CO3����������Ļ�ѧ����ʽΪ��2��_____ | ԭ��ɫ�����к���BaCO3 |
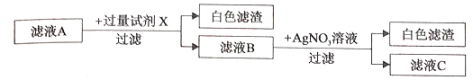
���𰸡�BaSO4 Na2CO3+2HC1=2NaCl+H2O+CO2�� ������CaCl2��Һ ��
��������
������Ŀ��������Ϣ��������ɫ����A�м������������ᣬ�����������ݲ�����������ʣ�࣬ԭ��ɫ�����к���BaSO4��
����������ɫ��ҺB�м������������ᣬ�����������ݲ�����ԭ��ɫ�����к���Na2CO3��̼���ƺ����ᷴӦ�����Ȼ��ƺ�ˮ�Ͷ�����̼����ƽ���ɣ�
����������ɫ��ҺB���ȼ����̪��Һ����Ϊ�˼�����ɫ��ҺB���Ƿ���OH-���Ӷ�֤��ԭ��ɫ�������Ƿ���Ba��OH��2��������Ҫ���������CaCl2��Һ��ȥ��ɫ��ҺB��NaCO3�����ĸ��ţ�Ȼ������Һ����ɫ���ж��Ƿ���Ba��OH��2����������ҺΪ��ɫ����֤����Ba��OH��2����������ҺΪ��ɫ����֤��û��Ba��OH��2����BaCO3��
��1����ɫ����A�м������������ᣬ�����������ݲ�����������ʣ�࣬ԭ��ɫ�����к���BaSO4����ΪBaSO4Ϊ��������Ĺ��壬�ʺ���BaSO4���ʴ�Ϊ��BaSO4��
��2��������ɫ��ҺB�м������������ᣬ�����������ݲ�����ԭ��ɫ�����к���Na2CO3��̼���ƺ����ᷴӦ�����Ȼ��ƺ�ˮ�Ͷ�����̼����ƽ���ɣ��ʴ�Ϊ��Na2CO3+2HCl=2NaCl+H2O+CO2����
��3��������ɫ��ҺB���ȼ����̪��Һ����Ϊ�˼�����ɫ��ҺB���Ƿ���OH-���Ӷ�֤��ԭ��ɫ�������Ƿ���Ba��OH��2��������Ҫ���������CaCl2��Һ��ȥ��ɫ��ҺB��NaCO3�����ĸ��ţ�Ȼ������Һ����ɫ���ж��Ƿ���Ba��OH��2����������ҺΪ��ɫ����֤����Ba��OH��2����������ҺΪ��ɫ����֤��û��Ba��OH��2����BaCO3���ʴ�Ϊ��������CaCl2��Һ���ޣ�