��Ŀ����
����Ŀ��������һ���ᣬ���ᾧ�壨H2C2O42H2O��������ˮ���۵�ϵͣ����Ȼ��ۻ��������ͷֽ⡣���ᣨH2C2O4�����������Ƶķ�Ӧ��H2C2O4+Ca(OH)2=CaC2O4��(��ɫ)+2H2O��
���������ۣ�
ʵ���ҿ��ü��Ȳ��ᾧ��ֽ�ķ������CO
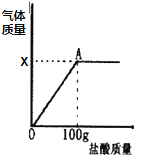
��1���ȼ��Ȳ��ᾧ������CO��CO2��H2O���仯ѧ����ʽ��_____��
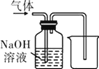
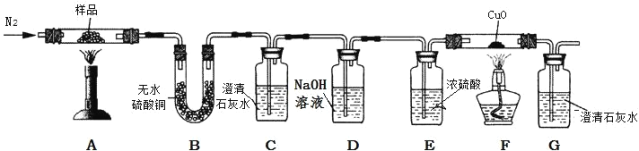
��2���������ͼװ���ռ�CO������Ӧ��_____�˽��루ѡ����a������b������
��ʵ�鷴˼��
��3������Ϊ��ͼ���Թܿ�Ӧ��������б����ʦ��ͬѧ���ۺ�һ����Ϊװ������ȷ�ģ�������_____��

��4��ʵ���й۲쵽����ʯ��ˮ����ǣ�����Ϊһ�����ɲ��ᾧ�����ȷֽ������CO2�����£�����Ϊ�ҵĽ��۲����ܣ�������_____��
���������ӣ�����Ӫ���ḻ�������˶���ͬʳ���ý�ʯ����Ҫ�ɷֲ���ƾ��壩��С��ͬѧ�Բ���ƾ�������ʼ���ɲ�������Ȥ��
������̽��������ͼװ�ý�����ƾ��壨CaC2O4��xH2O����Ʒ���¼��ȣ�ʹ����ȫ�ֽⲢ������������塣
���������ۣ�
��5��B�й۲쵽_____����˵����Ӧ������ˮ��
��6��C��G�г���ʯ��ˮ������ǣ�˵����Ӧ������_____��_____���塣
��7����ͬѧ��Ϊ�������۲��Ͻ��������ܵó���һ����̼���ɵĽ��ۡ���˵������_____��
�����ȷ����
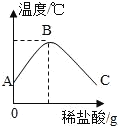
��8�������ȷ����ǶԲ���ƾ��壨CaC2O4��xH2O�������ȷֽ⣬���������ݣ����Ƴɹ����������ֽ��¶ȵĹ�ϵ��ͼ��

���¶�Ϊ200������ʱ������ȫ��ʧȥ�ᾧˮ�������нᾧˮ������Ϊ_____g��
�ڼ���CaC2O4��xH2O�е�x��CaC2O4����Է���������128����x=_____��
��800������ʱ���������������Ϊ�����ͼ��m��ֵ��_____��
��д������������12.8g��Ϊ10.0gʱ�Ļ�ѧ����ʽ_____��
���𰸡�H2C2O42H2O![]() CO��+CO2��+3H2O�� b �����۵�ϵͣ����ȷֽ�ʱ�����ۻ���ֽ������Թܿ���������б �����Dz�������������ʯ��ˮ�����ɲ���ư�ɫ���� ��ˮ����ͭ���� CO CO2 ������������û�н�������̼��ȫ���� 1.8 1 5.6 CaC2O4
CO��+CO2��+3H2O�� b �����۵�ϵͣ����ȷֽ�ʱ�����ۻ���ֽ������Թܿ���������б �����Dz�������������ʯ��ˮ�����ɲ���ư�ɫ���� ��ˮ����ͭ���� CO CO2 ������������û�н�������̼��ȫ���� 1.8 1 5.6 CaC2O4![]() CaCO3+CO��
CaCO3+CO��
��������
�������ۣ�
��1�����ݡ����Ȳ��ᾧ������CO��CO2��H2O�������仯ѧ����ʽ��H2C2O42H2O![]() CO��+CO2��+3H2O����
CO��+CO2��+3H2O����
��2��CO��������ˮ���ܶȱ�ˮС����ͼ1װ����ˮ���ռ�CO������Ӧ��b�˽��룻
ʵ�鷴˼��
��3������Ϊͼ2���Թܿ�Ӧ��������б��ԭ���Dz����۵�ϵͣ����ȷֽ�ʱ�����ۻ���ֽ⣬�����Թܿ���������б��
��4��ʵ���й۲쵽����ʯ��ˮ����ǣ�����Ϊһ�����ɲ��ᾧ�����ȷֽ������CO2�����£�����Ϊ�ҵĽ��۲����ܣ������Dz�����������������ᣨH2C2O4���������������Ʒ�Ӧ����CaC2O4������
��5������ͭ��ĩ��ˮ����ɫ��������ƾ��壨CaC2O4xH2O����Ʒ���¼��ȣ�B�й۲쵽����ͭ��ĩ��������˵����Ӧ������ˮ��
��6��C�г���ʯ��ˮ������ǣ�˵����Ӧ������CO2���壻G�г���ʯ��ˮ������ǣ�˵����Ӧ������CO���壬��Ϊһ����̼������ͭ��Ӧ�����ɶ�����̼��
��7����������ʵ�������֪��������ж�����̼���ɣ�����������������Һ������ʹ������̼��ȫ���գ�G�г���ʯ��ˮҲ�����ǣ�����D �� E װ֮��������һ��ʢ����ʯ��ˮ��ϴ��ƿ������ϴ��ƿ�г���ʯ��ˮ������ǣ���G�г���ʯ��ˮ�������˵������ֽ�����˶�����̼����ͬѧ��Ϊ�������۲��Ͻ��������ܵó���һ����̼���ɵĽ��ۡ����ɿ�����������û�н�������̼��ȫ���ա�
��8�����¶�Ϊ200������ʱ������ȫ��ʧȥ�ᾧˮ�������нᾧˮ������Ϊ14.6g-12.8g=1.8g��
�ڸ���ͼ���֪0��200���Ǿ���ʧȥ�ᾧˮ�Ĺ��̣�14.6��CaC2O4xH2Oʧȥˮ������12.8��CaC2O4��

![]() �����x�T1��
�����x�T1��
��800������ʱ���������������Ϊ��������������غ㶨�ɿ�֪���ù����������������ƣ������������Ƶ�����Ϊy��

![]() �����x=5.6g����ͼ��m��ֵ��5.6g��
�����x=5.6g����ͼ��m��ֵ��5.6g��
�ܸ���ͼ���֪800������ʱ����Ӧ�����������ƣ������ƿ�������̼��Ʒֽ����ã�400��ʱ����Ʒֽ⣬����������̼��ƣ�������̼��ƣ���10g�Ĺ���Ϊ̼��ƣ�10g̼��ƹ����У���Ԫ�ص�����=![]() ��̼Ԫ�ص�����=
��̼Ԫ�ص�����=![]() ����Ԫ�ص�����=10g-4g-1.2g=4.8g��12.8g������и�Ԫ�ص�����=
����Ԫ�ص�����=10g-4g-1.2g=4.8g��12.8g������и�Ԫ�ص�����=![]() ��̼Ԫ�ص�����=
��̼Ԫ�ص�����=![]() ����Ԫ�ص�����=12.8g-2.4g-4g=6.4g�����������غ㶨�ɿ�֪������Ʒֽ��IJ����г���̼����⣬����������̼Ԫ�ص�����=2.4g-1.2g=1.2g����Ԫ�ص�����=6.4g-4.8g=1.6g���������ʵ�����=1.2g+1.6g=2.8g���������������������ʵ�����=12.8g-10g=2.8g��ͬ�����������̼ԭ������ԭ�ӵĸ�����=
����Ԫ�ص�����=12.8g-2.4g-4g=6.4g�����������غ㶨�ɿ�֪������Ʒֽ��IJ����г���̼����⣬����������̼Ԫ�ص�����=2.4g-1.2g=1.2g����Ԫ�ص�����=6.4g-4.8g=1.6g���������ʵ�����=1.2g+1.6g=2.8g���������������������ʵ�����=12.8g-10g=2.8g��ͬ�����������̼ԭ������ԭ�ӵĸ�����=![]() �����Բ���Ʒֽ��IJ����г���̼����⣬����CO����Ӧ�Ļ�ѧ����ʽ�ǣ�CaC2O4
�����Բ���Ʒֽ��IJ����г���̼����⣬����CO����Ӧ�Ļ�ѧ����ʽ�ǣ�CaC2O4![]() CaCO3+CO����
CaCO3+CO����

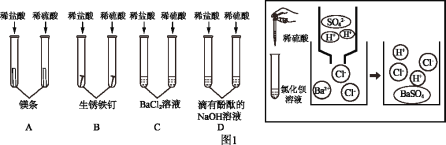
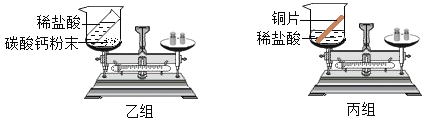
����Ŀ������ʵ����Ʋ��ܴﵽʵ��Ŀ�ĵ��ǣ� ��
ѡ�� | A | B | C | D |
ʵ��Ŀ�� | ��ȥCO�е�CO2��������CO | ̽����ȼ��ȼ����������� | ��ȥ����ͭ��ĩ�е�ͭ�� | ����NH4NO3��CuSO4��NaCl���ְ�ɫ��ĩ |
ʵ����� |
|
|
|
|
A. AB. BC. CD. D
����Ŀ������ʯ����һ����������������ҽҩ��ƿ����ѧ������ԭ�ϣ�ij��ѧ��ȤС�����ʯ�ۼ�����棬�����������̽����
���������ϣ�����ʯ����̼���ο������ʯ����Ҫ�ɷֵĻ�ѧʽ�ɱ�ʾΪ��xMgCO3 ��yCaCO3���������Ƕ������裬�䲻����ˮ��Ҳ�������ᷴӦ�����Ȳ��ֽ⣩
һ������̽��������ʯ�۵ijɷֺ�����
��ʵ�������
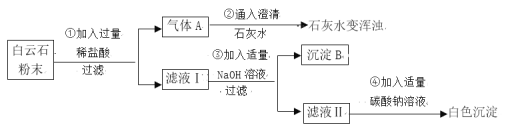
��1���ɲ���٢ڵ�����ɵó�����ʯ����һ�����е�������____________�������ӷ��ţ���
��2�����������������þ�Ļ�ѧ����ʽ��________________________________���÷�Ӧ����______����д��ѧ��Ӧ�������ͣ���
��3����Һ���е�������_________________________��
���� �ۣ��ۺ�����ʵ������ɳ���֤������ʯ��̼��ơ�̼��þ��ɡ�
��ʵ�����ɣ�С��ͬѧ����������Ϊ����������������ˮ�����³���B�г���������þ֮��Ӧ�û������������ƣ��Զ����ⶨ����ʯ�۵���ɸ��Žϴ�����ʦ��ָ���¸���ȤС������ȷֽⷨ���ж���̽����
��������̽��������ʯ�����
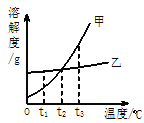
���������ϣ�̼��ƿ�ʼ�ֽ���¶�Ϊ898��,1000��ʱ��ȫ�ֽ�������ʯ�ҺͶ�����̼���壻̼��þ��̼��ƵĻ�ѧ�������ƣ�̼��þ��ʼ�ֽ���¶�Ϊ540�棬700��ʱ��ȫ�ֽ⡣
��ʵ����ƣ�Ϊ�ⶨ����ʯ�еĺ�������x��y��ֵ������ȤС�����������װ�ò�����ʵ�飺
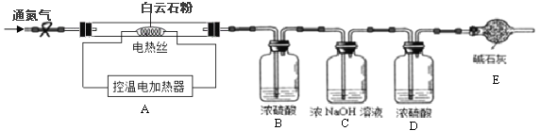
��ʵ�鲽�裩����װ���������װ�õ������ԣ���ȷ��ȡ15.0g ����ʯ��ĩ����Aװ���У����ɼУ�����һ��ʱ�䵪��������B��C��Dװ�õ��������۹رյ��ɼУ����µ�����������������¶���700�棬���������������������䣻�ܴ��ɼУ���������һ��ʱ�䵪����ȷ����B��C��Dװ�õ��������ݹرյ��ɼУ������¶���1000�棬���������������������䣬���ɼУ���������һ��ʱ�䵪����ȷ����B��C��Dװ�õ�������
ʵ�����ݼ�¼���±���
B����Һ����/g | C����Һ����/g | D����Һ����/g | |
��Ӧǰ | 50.0 | 100.0 | 50.0 |
700�� | 50.0 | 102.1 | 50.1 |
1000�� | 50.0 | 106.3 | 50.3 |
��ʵ����������ݴ�����
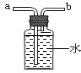
��4��װ��D��������______________��
��5�����������������������������һ��ʱ�䵪����Ŀ����_______________��
��6���������ʯ��̼��þ��������������д��������̣���_______________��
��7�����ʵ��֤������ʯ������ȫ�ֽ��ʵ�鷽����_____________________________��д��ʵ�鷽��������
��8������ʯ��xMgCO3 ��yCaCO3���е� x��y=___________����������ȣ���������ǰû�й���һ��ʱ��ĵ��������ᵼ��x��y��ֵ________����ƫ��ƫС�䣩��