��Ŀ����
������ͼʵ�飬�ش��������⡣


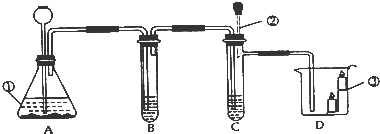
��1��д��Aװ���з�Ӧ�Ļ�ѧ����ʽ_______��
��2������ʯ����ҺȾ����ɫ�ĸ���ֽ�������뼯��ƿB�У��۲쵽��������_______��
��3��д��Cװ���з�Ӧ�Ļ�ѧ����ʽ_______������ˮ������Ϊ_______��
��4����ˮ����Dװ�ú�õ���ˮ��_______����������������
��5�����������в���ȷ����_______��


��1��д��Aװ���з�Ӧ�Ļ�ѧ����ʽ_______��
��2������ʯ����ҺȾ����ɫ�ĸ���ֽ�������뼯��ƿB�У��۲쵽��������_______��
��3��д��Cװ���з�Ӧ�Ļ�ѧ����ʽ_______������ˮ������Ϊ_______��
��4����ˮ����Dװ�ú�õ���ˮ��_______����������������
��5�����������в���ȷ����_______��
| A��ˮ����Ⱦ��Σ�����彡�� |
| B���������������õ���ˮϰ�� |
| C����ҵ��ˮ���������������ŷ� |
| D����ֹʹ��ũҩ�����ʣ��������ˮ����Ⱦ |
��1��2H2O  2H2��+ O2 ��
2H2��+ O2 ��
��2��ֽ������ɫ
��3��3Fe + 2O2 Fe3O4 ��ֹ�����ヲ��ʹ����ƿ��ը�ѡ�
Fe3O4 ��ֹ�����ヲ��ʹ����ƿ��ը�ѡ�
��4�������
��5��D
 2H2��+ O2 ��
2H2��+ O2 ����2��ֽ������ɫ
��3��3Fe + 2O2
 Fe3O4 ��ֹ�����ヲ��ʹ����ƿ��ը�ѡ�
Fe3O4 ��ֹ�����ヲ��ʹ����ƿ��ը�ѡ���4�������
��5��D
�����������Aװ��Ϊ���ˮװ�á��Ƹ����С������������̼�����ɫ����ʹʯ��Ⱦ�ɵ�С����ɫ����̼������Ƕ�����̼������˿ȼ�շų������ȣ����ƿ�в���ˮ��ƿ�ӻ�ը�ѡ��ȸ�װ��ֻ�ܳ�ȥ���������ʣ�����ˮ�����п��������ʣ����Ի��ǻ����ɻ��ʡ�ũҩ������Ĺ����൱������Ϊʹ�ò�������Ⱦˮ�����֮���á�

��ϰ��ϵ�д�
 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�
�����Ŀ

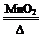 2KCl+3O2����
2KCl+3O2����


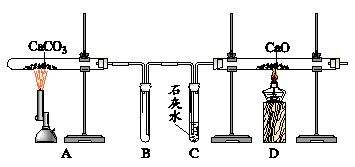
 CO��+CO2��+H2O
CO��+CO2��+H2O