��Ŀ����
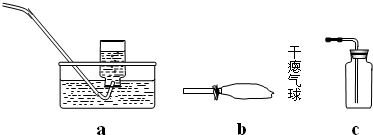
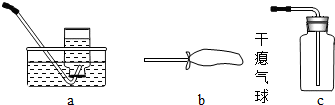
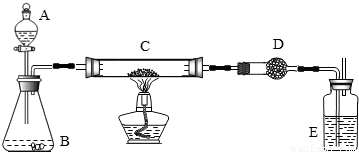
ij����С���ü���̼�ۣ�������������ͭ�Ļ����ķ����Ƶ�ͭ����Ʒ��������ͼװ�öԵõ���Ʒ����ʵ�飮ͼ������̨��װ������ȥ������������������ʵ�鱨�森
��һ��ʵ��Ŀ�ģ�
������ʵ����Ʒ��
��������ƽ����Һ©������ƿ��Ӳ�ʲ����ܡ�����ܡ��ƾ��ơ�ϴ��ƿ��
ҩƷ�����ɫͭ�ۣ���̿����Ʒ������������Һ���������̡���ʯ�ҡ�Ũ����
������ʵ������
| ʵ����� | ʵ������ | �йػ�ѧ����ʽ |
| ��C�м�����ƷWg��D��װ��ҩƷ�����Ϊm1g�����Ӻ��������װ�������ԣ� | ||
| ��A�Ļ����������μ���Һ | ||
| ��C���м��ȣ���C��ҩƷ��ַ�Ӧ�ر�A�Ļ�����ֹͣ���� | ||
| ��ȴ����D������Ϊm2g |
���壩��������ۣ�
ʵ����ɺ�ʦ����˵��������ʵ����ƣ���ʹC�з�Ӧ��ȫ��D��������ȫ�����õĽ��Ҳ�������������ۣ���ͬѧ�����B��C֮������һ��װ�ã��ٴ�ʵ��õ��˽�Ϊ��ȷ�Ľ������ԭʵ�����������ȷ��ԭ�������
��������һ������ʵ����̺�ʵ��װ�����ж�ʵ��Ŀ�ģ�
����������ͭ��̼�Ļ�ѧ���ʼ���װ�õ���;���ж�ÿһ����������д����ѧ����ʽ��
���ģ�����ͼ��װ�ü�ʵ��Ŀ�Ŀ���֪��Dװ�����������ն�����̼�ģ����Կ��Ը���̼Ԫ�ص������غ�����ɸ���Ľ��
���壩���ն�����̼�ü�ʯ�ң�����Ӧ��ʼ���ɵ���������һ����ˮ�������Ӷ�ʹ��Ӧ�������Dװ�õ��������ӣ����Ծݴ���ɸ���Ľ��
����������ͭ��̼�Ļ�ѧ���ʼ���װ�õ���;���ж�ÿһ����������д����ѧ����ʽ��
���ģ�����ͼ��װ�ü�ʵ��Ŀ�Ŀ���֪��Dװ�����������ն�����̼�ģ����Կ��Ը���̼Ԫ�ص������غ�����ɸ���Ľ��
���壩���ն�����̼�ü�ʯ�ң�����Ӧ��ʼ���ɵ���������һ����ˮ�������Ӷ�ʹ��Ӧ�������Dװ�õ��������ӣ����Ծݴ���ɸ���Ľ��
����⣺��һ��ʵ�����õ��ǹ�����̼������ԭ����ͭ�ģ������Ƶõ�ͭ�к���̼�ۣ���ô��ʵ���Ŀ�ľ�����̽��ͭ���ڻ�����е����������ģ�
����������ʵ��Ŀ�Ŀ���֪������֤ͭ��������������������������֮��Ӧ��Ȼ����������������㣬�������ж���B���������ķ�ӦΪ����������Һ�Ͷ������̵ķ�Ӧ�����Կ���֪����װ�����������ķ�ӦΪ��������Ͷ������̵ķ�Ӧ���ʿ���д����ѧ����ʽ����ʵ������Ϊ����B��Eװ���в������ݣ����ɵ�����ͨ�뱻���ȵ�̼�ۺ�ͭ�۵Ļ�����У�ͭ��̼�����������Ӧ���Կ���д���÷�Ӧ�Ļ�ѧ����ʽ������ԭ����ɫ��ͭ�ͺ�ɫ��̼��ɵĻ����Ҫȫ����Ϊ��ɫ��
���ģ����ݣ���������֪��D�������˶�����̼�����Կ���֪��D�ܵ������仯��Ϊ���ɵ����������̼�����������������غͶ��ɿ���֪����������̼�е�̼��������Ϊ̼�۵�����������̼������=��m2-m1����
��100%����ôͭ����������Ϊ��
��100%��
���壩����������Һ�ֽ�����������ݳ�ʱ�����һ����ˮ��������Dװ�����գ���ʹ��õĶ�����̼������������ӣ���̼���������ӣ����Ի�ʹ�������ƫС��Ϊ�˷�ֹˮ����Ӱ��ʵ������������B��C֮���һ��ʢ��Ũ�����ϴ��ƿ������ˮ�������Ӷ�ʹ��������ܵ�ȷ��
�ʴ�Ϊ����һ���ⶨͭ���ڻ�����е�����������
������
���ģ�
��100%��
���壩˫��ˮ�ֽ����������������һ����ˮ������ͨ��C��D���գ���ʹm2��ֵ����ʹ��õ�ͭ����������ƫС��ʢ��Ũ�����ϴ��ƿ��
����������ʵ��Ŀ�Ŀ���֪������֤ͭ��������������������������֮��Ӧ��Ȼ����������������㣬�������ж���B���������ķ�ӦΪ����������Һ�Ͷ������̵ķ�Ӧ�����Կ���֪����װ�����������ķ�ӦΪ��������Ͷ������̵ķ�Ӧ���ʿ���д����ѧ����ʽ����ʵ������Ϊ����B��Eװ���в������ݣ����ɵ�����ͨ�뱻���ȵ�̼�ۺ�ͭ�۵Ļ�����У�ͭ��̼�����������Ӧ���Կ���д���÷�Ӧ�Ļ�ѧ����ʽ������ԭ����ɫ��ͭ�ͺ�ɫ��̼��ɵĻ����Ҫȫ����Ϊ��ɫ��
���ģ����ݣ���������֪��D�������˶�����̼�����Կ���֪��D�ܵ������仯��Ϊ���ɵ����������̼�����������������غͶ��ɿ���֪����������̼�е�̼��������Ϊ̼�۵�����������̼������=��m2-m1����
| 12 |
| 44 |
W-(m2-m1)��
| ||
| W |
���壩����������Һ�ֽ�����������ݳ�ʱ�����һ����ˮ��������Dװ�����գ���ʹ��õĶ�����̼������������ӣ���̼���������ӣ����Ի�ʹ�������ƫС��Ϊ�˷�ֹˮ����Ӱ��ʵ������������B��C֮���һ��ʢ��Ũ�����ϴ��ƿ������ˮ�������Ӷ�ʹ��������ܵ�ȷ��
�ʴ�Ϊ����һ���ⶨͭ���ڻ�����е�����������
������
| ʵ����� | ʵ������ | �йػ�ѧ����ʽ | ||||||||
| ��C�м�����ƷWg��D��װ��ҩƷ�����Ϊm1g�����Ӻ��������װ�������ԣ� | ||||||||||
| ��A�Ļ����������μ���Һ | װ��B��E��������ð�� | 2H2O2
| ||||||||
| ��C���м��ȣ���C��ҩƷ��ַ�Ӧ�ر�A�Ļ�����ֹͣ���� | �Һ�ɫ�Ĺ�����Ϊ��ɫ | C+O2
2Cu+O2
| ||||||||
| ��ȴ����D������Ϊm2g |
W-(m2-m1)��
| ||
| W |
���壩˫��ˮ�ֽ����������������һ����ˮ������ͨ��C��D���գ���ʹm2��ֵ����ʹ��õ�ͭ����������ƫС��ʢ��Ũ�����ϴ��ƿ��
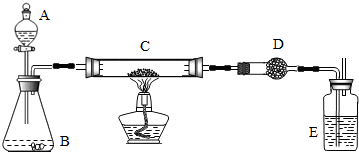
��������������̼��ͭ�Ļ�ѧ���ʣ�֪��������ʵ�����Ʒ�������д��ѧ����ʽ�������ù����Һ����ȡ����ķ���������������ʱ���״���ˮ���������ˮ�����ܹ�����ʵ������Ҫע���������и����ֹ����ʵ������

��ϰ��ϵ�д�
�����Ŀ
ij����С���ü���̼�ۣ�������������ͭ�Ļ����ķ����Ƶ�ͭ����Ʒ��������ͼװ�öԵõ���Ʒ����ʵ�飮ͼ������̨��װ������ȥ��
����������������ʵ�鱨�森
��һ��ʵ��Ŀ�ģ�______
������ʵ����Ʒ��
��������ƽ����Һ©������ƿ��Ӳ�ʲ����ܡ�����ܡ��ƾ��ơ�ϴ��ƿ��
ҩƷ�����ɫͭ�ۣ���̿����Ʒ������������Һ���������̡���ʯ�ҡ�Ũ����
������ʵ������
���ģ����㣺��Ʒ��ͭ����������=______���ú�W��m1\m2�Ĵ���ʽ��ʾ����
���壩��������ۣ�
ʵ����ɺ�ʦ����˵��������ʵ����ƣ���ʹC�з�Ӧ��ȫ��D��������ȫ�����õĽ��Ҳ�������������ۣ���ͬѧ�����B��C֮������һ��װ�ã��ٴ�ʵ��õ��˽�Ϊ��ȷ�Ľ���� ԭʵ�����������ȷ��ԭ�������______����B��C֮�����ӵ�װ�ú�����ʢ�ŵ�ҩƷ������______��
����������������ʵ�鱨�森

��һ��ʵ��Ŀ�ģ�______
������ʵ����Ʒ��
��������ƽ����Һ©������ƿ��Ӳ�ʲ����ܡ�����ܡ��ƾ��ơ�ϴ��ƿ��
ҩƷ�����ɫͭ�ۣ���̿����Ʒ������������Һ���������̡���ʯ�ҡ�Ũ����
������ʵ������
| ʵ����� | ʵ������ | �йػ�ѧ����ʽ |
| ��C�м�����ƷWg��D��װ��ҩƷ�����Ϊm1g�����Ӻ��������װ�������ԣ� | ||
| ��A�Ļ����������μ���Һ | ||
| ��C���м��ȣ���C��ҩƷ��ַ�Ӧ�ر�A�Ļ�����ֹͣ���� | ||
| ��ȴ����D������Ϊm2g |
���壩��������ۣ�
ʵ����ɺ�ʦ����˵��������ʵ����ƣ���ʹC�з�Ӧ��ȫ��D��������ȫ�����õĽ��Ҳ�������������ۣ���ͬѧ�����B��C֮������һ��װ�ã��ٴ�ʵ��õ��˽�Ϊ��ȷ�Ľ���� ԭʵ�����������ȷ��ԭ�������______����B��C֮�����ӵ�װ�ú�����ʢ�ŵ�ҩƷ������______��
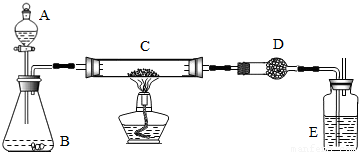
ij����С���ü���̼�ۣ�������������ͭ�Ļ����ķ����Ƶ�ͭ����Ʒ��������ͼװ�öԵõ���Ʒ����ʵ�飮ͼ������̨��װ������ȥ��
����������������ʵ�鱨�森
��һ��ʵ��Ŀ�ģ�______
������ʵ����Ʒ��
��������ƽ����Һ©������ƿ��Ӳ�ʲ����ܡ�����ܡ��ƾ��ơ�ϴ��ƿ��
ҩƷ�����ɫͭ�ۣ���̿����Ʒ������������Һ���������̡���ʯ�ҡ�Ũ����
������ʵ������
���ģ����㣺��Ʒ��ͭ����������=______���ú�W��m1\m2�Ĵ���ʽ��ʾ����
���壩��������ۣ�
ʵ����ɺ�ʦ����˵��������ʵ����ƣ���ʹC�з�Ӧ��ȫ��D��������ȫ�����õĽ��Ҳ�������������ۣ���ͬѧ�����B��C֮������һ��װ�ã��ٴ�ʵ��õ��˽�Ϊ��ȷ�Ľ���� ԭʵ�����������ȷ��ԭ�������______����B��C֮�����ӵ�װ�ú�����ʢ�ŵ�ҩƷ������______��
����������������ʵ�鱨�森

��һ��ʵ��Ŀ�ģ�______
������ʵ����Ʒ��
��������ƽ����Һ©������ƿ��Ӳ�ʲ����ܡ�����ܡ��ƾ��ơ�ϴ��ƿ��
ҩƷ�����ɫͭ�ۣ���̿����Ʒ������������Һ���������̡���ʯ�ҡ�Ũ����
������ʵ������
| ʵ����� | ʵ������ | �йػ�ѧ����ʽ |
| ��C�м�����ƷWg��D��װ��ҩƷ�����Ϊm1g�����Ӻ��������װ�������ԣ� | ||
| ��A�Ļ����������μ���Һ | ||
| ��C���м��ȣ���C��ҩƷ��ַ�Ӧ�ر�A�Ļ�����ֹͣ���� | ||
| ��ȴ����D������Ϊm2g |
���壩��������ۣ�
ʵ����ɺ�ʦ����˵��������ʵ����ƣ���ʹC�з�Ӧ��ȫ��D��������ȫ�����õĽ��Ҳ�������������ۣ���ͬѧ�����B��C֮������һ��װ�ã��ٴ�ʵ��õ��˽�Ϊ��ȷ�Ľ���� ԭʵ�����������ȷ��ԭ�������______����B��C֮�����ӵ�װ�ú�����ʢ�ŵ�ҩƷ������______��
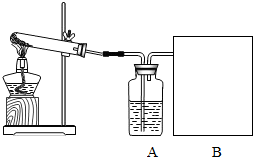
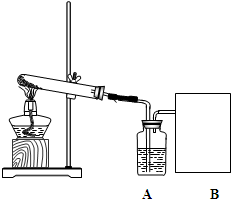 ��2006?����ij����С��������غͶ������̵Ļ������ȡ����ϴ�����������ʵ�鲽�����£�
��2006?����ij����С��������غͶ������̵Ļ������ȡ����ϴ�����������ʵ�鲽�����£�