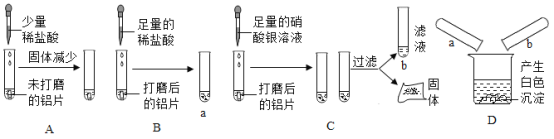
��Ŀ����
����Ŀ��ijͬѧ����֤�������ǵ���Ҫ�ɷ���̼���Σ����ռ����������塱��ʵ�顣��������·�������ʵ�飺
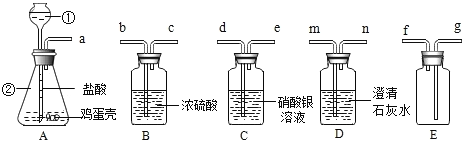
��1��д���������������ƣ���_____��_____
��2������A���������_____��
��3������������ѧ��֪ʶ������ΪAװ�ú�_____װ�����Ӳ�����ʲô����ʱ������˵�������ǵ���Ҫ�ɷ���̼���Σ�_____��д����װ���з�����Ӧ�Ļ�ѧ����ʽ��_____��
��4��д����Aװ�û�������ȡ���ճ�������һ�ֳ���������Ļ�ѧ����ʽ��_____����ָ������һ����Ҫ��;��_____��
���𰸡�����©�� ��ƿ �����Ǹ���Һ���ϣ����������ݲ������������ܽ� D Dװ���г���ʯ��ˮ����� CO2+Ca��OH��2��CaCO3��+H2O 2H2O2![]() 2H2O+O2�� ��������
2H2O+O2�� ��������
��������
��1��ʶ���������ʴ�Ϊ��������©��������ƿ��
��2��̼��ƺ����ᷴӦ�����Ȼ��ƺͶ�����̼��ˮ������̼��ƹ������٣�ͬʱ������ð�����ʴ�Ϊ�������Ǹ���Һ���ϣ����������ݲ������������ܽ⡣
��3��̼���κ����ᷴӦ���ɶ�����̼��������̼����ͨ�����ʯ��ˮ��ʯ��ˮ����ǣ��ʴ�Ϊ��D��Dװ���г���ʯ��ˮ����ǡ�������Ӧ�Ļ�ѧ����ʽ��CO2+Ca��OH��2��CaCO3��+H2O
��4��Aװ�����ڹ����Һ�岻������ȡ���壬�ʴ�Ϊ��2H2O2![]() 2H2O+O2��������������
2H2O+O2��������������
�𰸣�
��1��������©��������ƿ����2�������Ǹ���Һ���ϣ����������ݲ������������ܽ⣨3��D Dװ���г���ʯ��ˮ����� CO2+Ca��OH��2��CaCO3��+H2O��4��2H2O2![]() 2H2O+O2�� ��������
2H2O+O2�� ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ͬѧ�Ƕ�һ������������һ��ʱ�����504˫����������ܺ��棬���ʵ�����̽����

��������⣩����ijɷ���ʲô��
���������ϣ�(1)���������������ᷴӦ����Ӧ����Һ��ɫ�ʻ�ɫ������ʽΪFe2O3 +6HCl �T2FeCl3 + 3H2O
(2)�������Ȼ�����Һ�ڳ����·�����Ӧ�����Ȼ���������Ӧ�ķ���ʽΪ��Fe+2FeCl3�T3FeCl2
(3)���������׳���ʯ�ң������ƻ���ˮ��Ӧ���ų��������ȣ�����ʽΪCaO+ H2O�TCa��OH��2
���������룩�����Ϊ���ù����п��ܺ���Fe��CaO��Ca��OH��2��CaCO3
����Ϊ�����ܺ��е�������_____��д��ѧʽ��
��ʵ��̽������ͬѧ�ķ�����
ʵ����� | ʵ������ | ʵ����� |
��1��ȡ������������Թ��У�����������ˮ�ܽ⡣ | �����ܽ�ʱ�Թ���ڷ��̣��Թܵײ��в���� | ������һ������_____�� |
��2����ȡ������������Թ��У��μ�������_____�� | ��������ʧ���д�����ɫ���������������ͨ��ʯ��ˮ��ʯ��ˮ����ǣ����õ�dz��ɫ��Һ�� | ������һ����_____��_____ ʯ��ˮ����ǵķ���ʽ_____ |
��ʵ�����ɣ�ͨ����ͬѧ��ʵ�飬���������ʲ���ȷ��������_____��_____��
����Ŀ������ʵ������ܴﵽʵ��Ŀ����![]() ����
����![]()
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | ���������̼�л��е��������� | �������ǵ�ľ�������������� |
B | ��ȥCO2�е�HCl���� | ͨ������NaOH��Һ |
C | ��ȥ����������Һ�е�����ͭ | �������������ۣ����� |
D | ̽������������Һ��ϡ�����Ƿ�ǡ����ȫ��Ӧ | ��Ӧ�����Һ�еμ���ɫ��̪��Һ |
A. AB. BC. CD. D