��Ŀ����
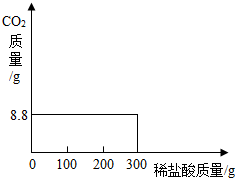
���ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õ�Na2CO3�Ậ��������NaCl��ij�о���ѧϰС���ȡ��NaCl��Na2CO3����25.0g���������Ƴ���Һ������������μ���������7.3%��ϡ������ǡ����ȫ��Ӧ�����ռ���8.8gCO2���壮����ԭ������Na2CO3�����������ͷ�Ӧ��������Һ����������������������̼���������ᷴӦ�ų�������̼���Ȼ��Ʋ������ᷴӦ�����ݷ�Ӧ�Ļ�ѧ����ʽ�������ɶ�����̼�������ɼ�����Ʒ��̼���Ƶ�������̼���Ƶ���������Ʒ�����ıȿɼ���ԭ������Na2CO3������������
��Ӧ��������ҺΪ�Ȼ�����Һ�������Ȼ�����ԭ��Ʒ���Ȼ��ƺͷ�Ӧ�����Ȼ�������������ɣ������ɶ�����̼�����ɼ�������ɵ��Ȼ��Ƶ���������Ʒ������̼������������Ϊ��Ʒ���Ȼ��Ƶ��������������Ȼ��Ƶ���������������Һ�������ȿɼ��㷴Ӧ��������Һ�������������������з�Ӧ��������Һ���������������غ㶨�ɼ�����ã�
��Ӧ��������ҺΪ�Ȼ�����Һ�������Ȼ�����ԭ��Ʒ���Ȼ��ƺͷ�Ӧ�����Ȼ�������������ɣ������ɶ�����̼�����ɼ�������ɵ��Ȼ��Ƶ���������Ʒ������̼������������Ϊ��Ʒ���Ȼ��Ƶ��������������Ȼ��Ƶ���������������Һ�������ȿɼ��㷴Ӧ��������Һ�������������������з�Ӧ��������Һ���������������غ㶨�ɼ�����ã�
����⣺����Ʒ��̼���Ƶ�����Ϊx�����ɶ�����̼8.8g�������Ȼ��Ƶ�����Ϊy������HCl������Ϊz��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 117 44
x z y 8.8g
=
x=21.2g
=
y=23.4g
=
z=14.6g
ԭ������Na2CO3����������=
��100%=84.8%
����ϡ���������=14.6��7.3%=200g
��Ӧ��������Һ��������������=
��100%=12.6%
��ԭ������Na2CO3����������Ϊ84.8%����Ӧ��������Һ��������������Ϊ12.6%��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 117 44
x z y 8.8g
| 106 |
| x |
| 44 |
| 8.8g |
| 117 |
| y |
| 44 |
| 8.8g |
| 73 |
| z |
| 44 |
| 8.8g |
ԭ������Na2CO3����������=
| 21.2g |
| 25.0g |
����ϡ���������=14.6��7.3%=200g
��Ӧ��������Һ��������������=
| 25.0g-21.2g+23.4g |
| 25.0g+200g-8.8g |
��ԭ������Na2CO3����������Ϊ84.8%����Ӧ��������Һ��������������Ϊ12.6%��
���������ݻ�ѧ����ʽ���Ա�ʾ��Ӧ�и����ʵ������ȣ��ɷ�Ӧ���������ʵ������ɼ������Ӧ���������ʵ�������

��ϰ��ϵ�д�
�����Ŀ
 27����Һ�������Ϳ����о��й㷺����;����ش��������⣺
27����Һ�������Ϳ����о��й㷺����;����ش��������⣺
 ��2003?���ݣ����ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õ�Na2CO3�Ậ��������NaCl��
��2003?���ݣ����ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õ�Na2CO3�Ậ��������NaCl��