��Ŀ����
��5�֣��й����������ı�����2014��3��30�շ��ɱ������Ϲ����ʺ���ͥ�ݽ���ν���Ϻ���Ȩ�ٲ������з���ʾ�������ٲá��Ϻ����ҹ��Ĺ����캣���Ϻ��̲��ŷḻ�ĺ�����Դ��
��1���Ϻ������̺��������Ϳ����Դ������ȼ������������Ȼ������Ҫ�ɷ�CH4����ˮ�γɵı�״���壬����ȼ�գ���һ�������Դ��д��CH4ȼ�յĻ�ѧ����ʽ
������ ��Ӧ���͡����ӡ��û����������ֽ⡱�������ϡ�������������ѡ��
��2���ҹ���ѧ��ѧ�Һ�°����ĺ����Ƽ�������Ժ�ˮ��ɹ�Ρ��õ����Ȼ���Ϊԭ���Ƶ�Na2CO3��
Na2CO3��NaCl���ܽ��������ͼ��ʾ���ش��������⣺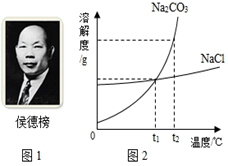
��t1��ʱ��Na2CO3������Һ��������������
NaCl �ġ�������ڡ���С�ڡ������ڡ�֮һ��
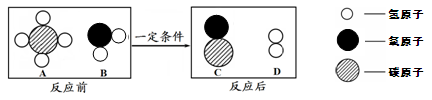
���������μ��������������һ���������ɹ�Σ������̼�������ܽ�����ߣ�˵����������ԭ������
����
��1��CH4 + 2O2��ȼCO2+ 2H2O ����
��2�� �ٵ��� ��̼���Ƶ��ܽ�����¶����߶����������Ȼ��Ƶ��ܽ����¶ȵı仯Ӱ�첻�������¶ȸߣ�����ǿ�������������ᾧ��ʳ�Σ���̼���������ܽ�ȱ������������������¶ȵͣ�̼���������ܽ�����Լ�С���ᾧ������ʳ�ε��ܽ�ȱ仯�������������
���������������1��CH4ȼ�յĻ�ѧ����ʽ��CH4 + 2O2��ȼCO2+ 2H2O������������Ӧ����
��2���ٴ��ܽ�����߿�֪��t1��ʱNa2CO3��NaCl���ܽ�������ཻһ�㣬�����ߵ��ܽ����ȣ�����Na2CO3������Һ������������������NaCl �ģ����������μ��������������һ���������ɹ�Σ������̼������ܽ�����ߣ�ԭ���ǣ�̼���Ƶ��ܽ�����¶����߶����������Ȼ��Ƶ��ܽ����¶ȵı仯Ӱ�첻�������¶ȸߣ�����ǿ�������������ᾧ��ʳ�Σ���̼���������ܽ�ȱ������������������¶ȵͣ�̼���������ܽ�����Լ�С���ᾧ������ʳ�ε��ܽ�ȱ仯�������������
���㣺����ȼ�յķ���ʽ���ܽ�����ߵ�����

 53���ò�ϵ�д�
53���ò�ϵ�д���9�֣���2013?����һģ��С���Ķ��������ϵ�֪������صķֽ���ö������̡�����ͭ�����������������ǣ�����Ӱ������طֽ�����ؼ������Ĵ�Ч��������̽����Ȥ��
��������⡿����ͭ�Ƿ�ȶ������̴�Ч�����ã�Ӱ������طֽ����ʵ���������Щ�أ�
�����ʵ�顿С�������ɵ����������Ϊ������������м���ʵ�飮
| ��� | KClO3������ | �������ʵ����� | �¶� | ��������� | ��Ӧ����ʱ�� |
| �� | 2.0g | | 330�� | 100mL | t1 |
| �� | 2.0g | CuO 0.5g | 330�� | 100mL | t2 |
| �� | 2.0g | MnO2 0.5g | 330�� | 100mL | t3 |
| �� | 2.0g | MnO2��_________�� g | 380�� | 100mL | t4 |
��2������ΪС�����ʵ��ۺ�ʵ��ڶԱȵ�Ŀ������_________����
��3��ʵ�����MnO2������Ϊ��_________��g����t3��t4����ѧ��Ӧ�������¶ȵĹ�ϵ����_________����
��4��д��ʵ������漰�Ļ�ѧ����ʽ��_________����
���������У����������ӵ���
| A��ˮ | B��Һ�� | C�������� | D��������̼ |