��Ŀ����
����Ŀ����װ�е���ˮ��A��B��C�ձ��зֱ����10g��25g��25gNaNO3���壬����ܽ��������ͼһ��ʾ��
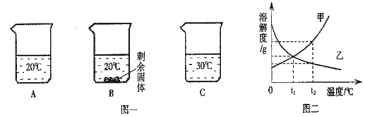
(1)ͼ���ܱ�ʾNaNO3�ܽ�����ߵ���_________(����������������)��
(2)��ͼ�����������ֱ�100g�ס��ҵı�����Һ��t2�潵�µ�t1�棬��������Һ��������ȷ��______(�����)��
A �ס��Ҷ��DZ�����Һ B �����ܼ��������ף��� C ��Һ�������ף��� D���������������ף���
���𰸡��� BD
��������
(1) ����ͼһ��֪�������Ƶ��ܽ�����¶ȵ����߶���������ͼ�����ܱ�ʾNaNO3�ܽ�����ߵ��Ǽף�
(2) A�����ڼ��ܽ�����¶ȵĽ��Ͷ���С��������ı�����Һ���º���о�������������Һ��Ϊ������Һ�����ҵ��ܽ�����¶ȵĽ��Ͷ�����������ı�����Һ���º�ͻ��ɲ�������Һ����A����
B�������ڽ��¹������ܼ���������û�з����仯�����ֻҪ�Ƚϳ�����ԭ�����ܼ��Ķ��ټ��ɣ������������ʵ��ܽ�����߿�֪����t2��ʱ�����ܽ�ȴ����ҵ��ܽ�ȣ���������Һ�����ʵ����������ף��ң��������ı�����Һ�����ʵ������ף��ң����ܼ��ף��ң���B��ȷ��
C�����ڼ���Һ�����¶ȵĽ��ͻ��о��������������Һ�������С�����ҵ���Һ��û�о���������Һ�������ᷢ���仯����˽��º���Һ�������ף��ң���C����
D����t1��ʱ�������ʵ��ܽ����ȣ��������ʵı�����Һ�����ʵ�����������ȣ�������Һ�Ѿ������DZ�����Һ�������Һ�����ʵ����������ף��ң���D��ȷ����ѡBD��

 53������ϵ�д�
53������ϵ�д�����Ŀ��ij��ѧ��ȤС��Բ���ʹ�õĽ���ɷֲ�����Ũ����Ȥ�����뵽�������������ԣ�����ҺpH______![]() ��������������С��������������
��������������С��������������![]() �������ʵ��
�������ʵ��![]() �����ò�pH�ķ���
�����ò�pH�ķ���![]() ֤����������������______��������Ͱ�г������Һ����ɫ���Ը�����ͬѧ����������̽����
֤����������������______��������Ͱ�г������Һ����ɫ���Ը�����ͬѧ����������̽����
��������⣩�������Ҫ�ɷ���ʲô��
����������裩
����٣�����ֻ�����
����ڣ�����ֻ������ͭ��
����ۣ����ܺ������������ͭ��
���в���______�����ͬѧ���ԣ�ԭ����______��
��ʵ��̽����Ϊ����֤�����������룬ͬѧ�����������ʵ�����̽����
ʵ����� | ʵ������ | ʵ����� |
ȡ���������������������ˮ�����Һ����ȡ2mL��õ���Һ��һ֧�ྻ�Թ��У�����������μ�����������������Һ | __ | ���� |
д������ʵ���з����Ļ�ѧ����ʽ______![]() д��һ������
д��һ������![]() ��
��
����Ŀ��̼��������������ɼ�����Ҫ�ɷ�֮һ��
С��ͬѧ�о���Ӧ2NaHCO3+H2SO4=Na2SO4+2H2O+2CO2����NaHCO3��CO2֮�����Ĺ�ϵ��
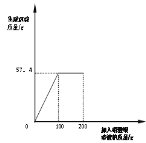
��1�����ۼ��㣺1.26gNaHCO3��ĩ������ϡ���ᷴӦ����������CO2������______��д��������̣���
��2��ʵ��С������ͼװ�ý���ʵ�飬��ʢ������ϡ�������ƿ�м���1.26g NaHCO3��ĩ����ȫ��Ӧ�����ٲ������ݡ���ȡ��Ӧǰ���������±������ַ�Ӧǰ�������仯ֵ____����������������С��������������������CO2����������ֵ��ԭ�������____��

��Ӧǰ | ��Ӧ�� | |
NaHCO3/g | ��ƿ+ϡ����/g | ��ƿ+��Ӧ����Һ/g |
1.26 | 24.59 | 25.36 |