��Ŀ����
 Ϊ�˳���������������ڼ�ʹ�ú���̼��Ƶġ�ʯͷֽ����ij�о�С��Ϊ�˲ⶨ����̼���0.88
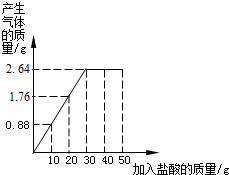
Ϊ�˳���������������ڼ�ʹ�ú���̼��Ƶġ�ʯͷֽ����ij�о�С��Ϊ�˲ⶨ����̼���0.88������������ȡ10g��Ʒ�����ձ��У���50gϡ��������ɴμ����ձ��У���¼��Ӧ���̵�������ϵ��ͼ��ʾ��
��1����������������Ϊ
30
30
gʱǡ����ȫ��Ӧ����ʱ��������2.64
2.64
g����2��10g��Ʒ��̼��Ƶ����������Ƕ��٣�
��3������ϡ���������ʵ����������Ƕ��٣�
��������ͼ��֪������ɶ�����̼������Ϊ2.64g�����Ը��ݷ�Ӧ�Ļ�ѧ����ʽ���ɷų�������̼������������Ʒ��̼��Ƶ����������������Ʒ��̼��Ƶ����������Լ�����ϡ���������ʵ�����������
����⣺��1����ͼ��֪������ɶ�����̼������Ϊ2.64g����ʱ�������������Ϊ30g��
��2����̼�����Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 2.64g
=
x=6g
10g��Ʒ��̼��Ƶ���������=
��100%=60%
��10g��Ʒ��̼��Ƶ���������Ϊ60%��
��3����30g ϡ���������ʵ�����Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
73 44
y 2.64g
=
y=4.38g
����ϡ���������ʵ���������=
��100%=14.6%
������ϡ���������ʵ�����������14.6%��
�ʴ�Ϊ��
��1��30g��2.64g��
��2��60%����3��14.6%��
��2����̼�����Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 2.64g
| 100 |
| x |
| 44 |
| 2.64g |
x=6g
10g��Ʒ��̼��Ƶ���������=
| 6g |
| 10g |
��10g��Ʒ��̼��Ƶ���������Ϊ60%��
��3����30g ϡ���������ʵ�����Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
73 44
y 2.64g
| 73 |
| y |
| 44 |
| 2.64g |
y=4.38g
����ϡ���������ʵ���������=
| 4.38g |
| 30g |
������ϡ���������ʵ�����������14.6%��
�ʴ�Ϊ��
��1��30g��2.64g��
��2��60%����3��14.6%��
���������������غ㶨�ɣ�����ÿ�μ���ϡ������ձ����ձ��������������ı仯�����Եó�ÿ��ʵ�����ϡ�������ų�������̼���������ݴ˶Է�Ӧ��������жϣ�

��ϰ��ϵ�д�
�����Ŀ
 Ϊ�˳���������������ڼ�ʹ�ú���̼��Ƶġ�ʯͷֽ����ij�о�С��Ϊ�˲ⶨ����̼���0.88
Ϊ�˳���������������ڼ�ʹ�ú���̼��Ƶġ�ʯͷֽ����ij�о�С��Ϊ�˲ⶨ����̼���0.88