��Ŀ����
����Ŀ����ѧ�����ǵ�����������ϢϢ��ء���ش��������⡣
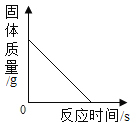
��1��ʯī���ռ����ʯ�����������У��������缫����_____________�������ڸ���������������_______��
��2��ʳ����������������Ԥ��ƶѪ�����е���������ָ__________��ѡ����Ԫ��������ԭ������
��3��������ˮ��Ӳˮ����ˮ������������ˮ�ķ����ǣ��ֱ�ȡ����������ˮ���������������___________�����۲������ĭ�Ķ���
��4��������Ҫͨ����ʯȼ��ȼ�ջ�ȡ�����������Ļ�ʯȼ�ϳ���Ȼ����ú�⣬����__________����Ȼ���еļ���ȼ�յĻ�ѧ����ʽΪ______________��
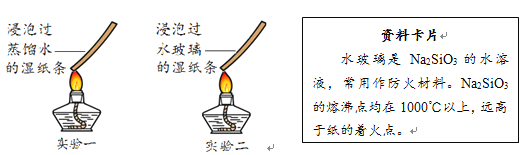
��5��������ÿ������ʴ�����ϵĽ����豸�Ͳ��ϣ��ߴ��������20%~40%���������������__________����ֹ�������DZ���������Դ��һ����Ч;������ֹ�������һ�־��巽����___________��
��6�����ֶ�������ͷ�����������֣�ԭ����________��ѡ����ţ���
���ṩ��ȼ�� ���ṩ���� ��ʹ��ȼ����¶ȴﵽ�Ż��
һ���������֣�������Ա����ˮ����𣬸÷������ݵ���Ҫ���ԭ��_____________��
���𰸡�ʯī ��ʯ�� Ԫ�� ����ˮ ʯ�� 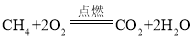 ����������ˮͬʱ�Ӵ� ˢ�ᣨ�������ɣ� �� ʹ��ȼ���¶Ƚ����Ż������
����������ˮͬʱ�Ӵ� ˢ�ᣨ�������ɣ� �� ʹ��ȼ���¶Ƚ����Ż������
��������
��1��ʯī���е����ԣ��ռ����ʯ��û�е����ԣ�ʯīû�м��ԣ��ռ���м��Ե��н�ǿ�ĸ�ʴ�ԣ���ʯ�Ҿ��м��ԡ�ʯī���ռ����ʯ�����������У��������缫����ʯī�������ڸ�����������������ʯ�ҡ�
��2����������Ԫ����ɡ�ʳ�á��������͡���Ԥ��ƶѪ�����еġ�������ָԪ�أ�
��3��������ˮ��Ӳˮ����ˮ������������ˮ�ķ����ǣ��ֱ�ȡ����������ˮ��������������ķ���ˮ�����۲������ĭ�Ķ��٣�������ĭ�������ˮ��������ĭ�ٵ���Ӳˮ��
��4��������Ҫͨ����ʯȼ��ȼ�ջ�ȡ�����������Ļ�ʯȼ�ϳ���Ȼ����ú�⣬����ʯ�ͣ���Ȼ���еļ���ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ�� ��
��
��5�������������������������ˮͬʱ�Ӵ�����ֹ�������DZ���������Դ��һ����Ч;������ֹ������ľ��巽����ˢ�ᣨ�������ɣ�
��6�����ֶ�������ͷ�����������֣�ԭ����ʹ��ȼ����¶ȴﵽ�Ż�㣻��ѡ�ۡ�ˮ�������ȣ���ʹ��ȼ����¶Ƚ��͡�һ���������֣�������Ա����ˮ����𣬸÷������ݵ���Ҫ���ԭ��ʹ��ȼ���¶Ƚ����Ż�����¡�

 ��ѧȫ��������ѵ��ϵ�д�
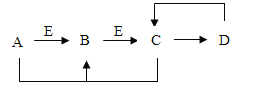
��ѧȫ��������ѵ��ϵ�д�����Ŀ������ͼ������ȷ��ӳ��Ӧ��ϵ���ǣ�������
|
|
|
|
A���õ���������Ũ�ȵĹ���������Һ��ȡ���� | B����ˮͨ��һ��ʱ�� | C��һ�������ĺ������ܱ�������ȼ�� | D������һ������������������� |
A. A B. B C. C D. D
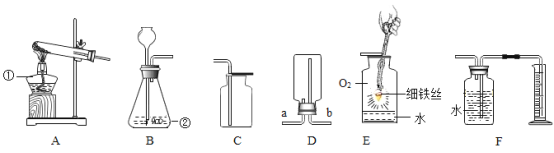
����Ŀ����1������ͼʾʵ��װ�ã��ش��������⡣
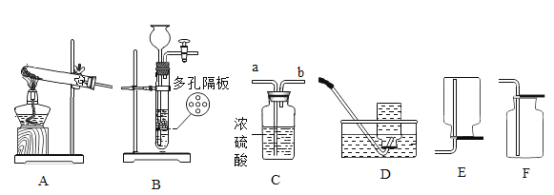
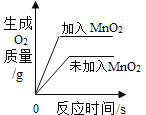
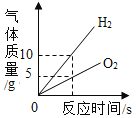
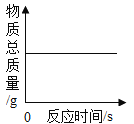
��ʵ�����ø��������ȡ����������װ��Ӧѡ������װ����___________�����ţ������ù���������Һ��ȡ�����������װ�ú���������˳��Ϊ������װ����C��___________�����ţ�������װ��ʱ������װ�õij�����Ӧ��װ��C��_____________������a������b������������
��ʵ����ʹ��Bװ����ȡ������̼��ͻ���ŵ���____________�����ƵõĶ�����̼ͨ����ɫʯ����Һ�У��۲쵽��������_____________��
��2��ij��ѧ��ȤС����ʯ��ʯ��������ȡһƿCO2������������ʯ��ˮ���뼯��ƿ�У�����û�б���ǡ���ȤС�������쳣���������̽����
��������⣩����ʯ��ˮΪʲôû�б���ǣ�
����������]��CO2����ʱ��CaCO3������ת��Ϊ������ˮ��Ca��HCO3��2��
��AgCl������ϡ����
��������룩I.ʯ��ˮ����ȫ���� ��_______.��.CO2�л���HCl
��ʵ�鷽������ȤС����ԭҩƷ������ȡCO2��������̽����
�Ʊ�CO2�Ļ�ѧ����ʽΪ___________��
ʵ�鲽�� | ʵ������ | ʵ����� |
1.ȡ��������ʯ��ˮ���Թ��У�����________��Һ�� | ������ɫ���� | ����I������ |
2.����������ͨ��ʢ�г���ʯ��ˮ���Թ��� | __________ | ������������ |
3.____________ | ______________ | ���������� |