��Ŀ����
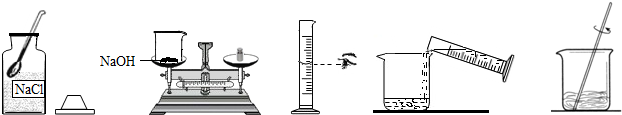
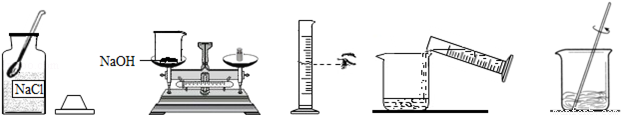
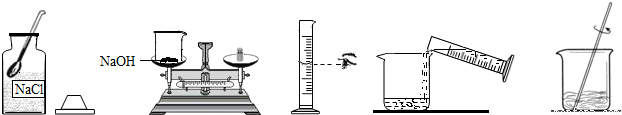
С����Ҫ����80g��������Ϊ10%������������Һ��������Ҷ����ǩ������ͼ�����Ƹ�����������Һ��ʵ�����ʾ��ͼ��
��ʵ�鲽�衿
��1�����㣺��Ҫ�������ƹ�������g��ˮ����mL��ˮ���ܶ���1.0g/cm3�ƣ�
��2����������������ƽ��ȡ�������ƹ��壬�ù��Ϊ�������10mL������50mL����100mL��������Ͳȡ����Ҫ��ˮ������ʢ���������Ƶ��ձ��У�
��3���ܽ⣺�ò��������裬ʹ�������ƹ�����ȫ�ܽ⣮
����չ˼ά��
����С�������������Ƶ�����������Һ��ȫ�к�������������Ϊ10%�����ᣬ������������������Ƕ��٣�
����Ӧ�Ļ�ѧ����ʽΪ��NaOH+HCl=NaCl+H2O��
| ��1��������������=��Һ���������ʵ������������ɸ�����Һ�����������ʵ�������������������Һ����Ҫ�����ʵ��������ٸ����ܼ�����=��Һ����﹣�������������������ˮ�������� ��2���Ӽ�Сʵ�����ĽǶ�ȥѡ����Ͳ�����̣� ��3��ϡ����������������Һ��Ӧ�����Ȼ��ƺ�ˮ�����ݲμӷ�Ӧ���������Ƶ��������ɷ�Ӧ�Ļ�ѧ����ʽ������μӷ�Ӧ��������������ɣ� | |
| ��� | �⣺��1����������=��Һ���������ʵ���������������80g��������Ϊ10%������������Һ�����������Ƶ�����=80g��10%=8g���ܼ� ��2��ѡȡ��Ͳʱ������ѡ����һ����ȡ����С������Ͳ��Ӧ��100mL��Ͳ��ȡ72mLˮ�� ��3���������������������Ϊx�� NaOH+HCl=NaCl+H2O 40 36.5 8g 10%x
�ʴ�Ϊ����1��8��72����2��100mL����3���������������������73g�� |
��

 ��У����ϵ�д�
��У����ϵ�д� ����=��Һ����﹣��������������������ˮ������=80g﹣8g=72g��ˮ���ܶ�Ϊ1g/cm3�������V=
����=��Һ����﹣��������������������ˮ������=80g﹣8g=72g��ˮ���ܶ�Ϊ1g/cm3�������V= =
=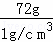 =72cm3=72mL��
=72cm3=72mL��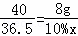 ��x=73g
��x=73g