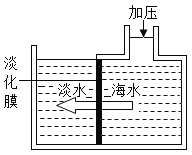
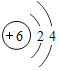��Ŀ����
����Ŀ���й��Ϻ��Ϻ������־�ġ���ȼ������Դ������Ȼ�����ܵ�����Դ��ԼΪ16���������ף�ռ�й���������Դ��������֮һ������70���̲���153��7��ƽ������������
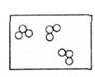
��1����ȼ���ֳ���Ȼ��ˮ������ں��ĸ�ѹ�������������γɵĽᾧˮ���������������1�����ȼ���ɴ���100-200�������Ȼ������Ȼ��ȼ��ʱ����_____ɫ���棬����ѧ��ת��Ϊ_____�ܣ���д����Ȼ����ȫȼ��ʱ�Ļ�ѧ����ʽΪ_____��
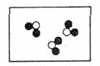
��2���ڿ�ȼ�������У�ƽ��ÿ46��ˮ���ӹ���8������ÿ����������һ��������ӻ�һ�������ˮ���ӣ���ÿ8��������6�������˼�����ӣ�����2����2�������ˮ��������䣬���ȼ����ƽ����ɿ��Ա�ʾΪ��______��
A CH4��2H2O B CH4��6H2O C CH4��8H2O D CH4��10H2O
��3����ȼ������Ϊ����ʮһ���������Դ��������Ϊ��ȼ���ŵ���_____��Ҳ������Ϊ��ȼ���Ŀ���������һ��˫�н�����Ҫ���ضԴ�����������_____��
��4���Ϻ���ȼ�����ķ���Ϊ�ҹ���ʹ�ø�Ч������Դ������ʮ�ֹ����Ŀ���ǰ��������Ϊ�ܿ������õ�����Դ���У�������д���֣�_____��
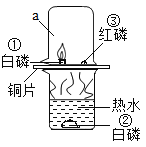
���𰸡��� �� 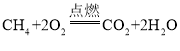 C �����Ի����Ѻá�������Ϊ��̬�����䣬��ʱ��Һ�� ��ȼ���еļ�������ӷ��������У������ȫ������ЧӦ ̫���ܡ����ܡ������ܡ���ϫ�ܵ�
C �����Ի����Ѻá�������Ϊ��̬�����䣬��ʱ��Һ�� ��ȼ���еļ�������ӷ��������У������ȫ������ЧӦ ̫���ܡ����ܡ������ܡ���ϫ�ܵ�
��������
��1����Ȼ��ȼ��ʱ������ɫ���棬����������н���ѧ��ת��Ϊ���ܣ�ȼ��ʱ���ɶ�����̼��ˮ����ѧ����ʽΪ��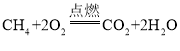 ���ʴ�Ϊ�������ȣ�
���ʴ�Ϊ�������ȣ�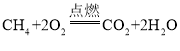
��2����Ϊƽ��ÿ46��ˮ���ӹ���8������������ÿ8��������6��������CH4���ӣ�����2����2�������H2O��������䣻������Ȼ��ˮ����Ĺ����к�6��CH4���ӡ�46+2=48��H2O���ӣ���CH4������H2O������������Ϊ6:48=1:8�����ж���Ȼ��ˮ�����ƽ����ɿɱ�ʾΪCH48H2O���ʴ�ѡC��
��3����ȼ���ĺô����ڴ����Ի����Ѻá�������Ϊ��̬�����䣬��ʱ��Һ�����������������ڿ��������п�ȼ���еļ�������ӷ��������У������ȫ������ЧӦ���ʴ�Ϊ�������Ի����Ѻá�������Ϊ��̬�����䣬��ʱ��Һ������ȼ���еļ�������ӷ��������У������ȫ������ЧӦ��
��4�����Կ���������Դ�кܶ࣬�磺̫���ܡ����ܡ������ܡ���ϫ�ܵȣ��ʴ�Ϊ��̫���ܡ����ܡ������ܡ���ϫ�ܵȡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ij��ֽ���ŷŵķ�ˮ�к���Na2CO3��NaOH��Ϊ�ⶨ��ˮ��Na2CO3������������ȡ50g��ˮ��Ʒ���ձ��У���40gϡ����ֳ�4�ȷݷ�4�λ������뵽�ձ��У���������������ϡ������Һ��������ϵ��ͼ������ʾ���ش��������⣺
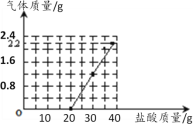
��1������ϸ������ͼ���ݣ�����±�ʵ���¼��
ʵ����� | 1 | 2 | 3 | 4 |
ϡ�����������g�� | 10 | 10 | 10 | 10 |
��������������g�� | 0 | ____ | ____ | ____ |
��2��������������________ g��
��3������÷�ˮ��Na2CO3����������________��
��4���ⶨNa2CO3������������Ҳ�������з�������ȡ������ˮ��Ʒ50g�����ˮ����μ���CaCl2��Һ������Ӧ��ȫʱ�������ˡ�ϴ�ӡ����_________�����ɼ����Na2CO3������������