��Ŀ����
ˮ�DZ������Ȼ��Դ���������������ũҵ������������Ҫ���塣
�ٱ���ˮ�Ļ�ѧ���ʵ����� ��9�� (������)����90��ˮ�к��� ��10�� ����ԭ�ӣ�
���ܹ�֤��ˮ����Ԫ�غ���Ԫ����ɵ�ʵ���� ��11�� ���û�ѧ����ʽ��ʾ����
����ʢ����ɫʯ����Һ���Թ���ͨ�������̼��ʯ���� ��12�� ɫ�������Ļ�ѧ��Ӧ����ʽ�� ��13�� ��
��ʵ����Ҫ����50��20%���������Һ����Ҫ��14�� ������أ���15�� ����ˮ��ʵ���г��˵�����ƽ�����ʹ�õ������� ��16�� ��
�ٱ���ˮ�Ļ�ѧ���ʵ����� ��9�� (������)����90��ˮ�к��� ��10�� ����ԭ�ӣ�
���ܹ�֤��ˮ����Ԫ�غ���Ԫ����ɵ�ʵ���� ��11�� ���û�ѧ����ʽ��ʾ����
����ʢ����ɫʯ����Һ���Թ���ͨ�������̼��ʯ���� ��12�� ɫ�������Ļ�ѧ��Ӧ����ʽ�� ��13�� ��
��ʵ����Ҫ����50��20%���������Һ����Ҫ��14�� ������أ���15�� ����ˮ��ʵ���г��˵�����ƽ�����ʹ�õ������� ��16�� ��
�٣�9��ˮ���ӣ�д���Ų����֣�
�ڣ�10��6.02��1024
 �ۣ�11��2H2O
�ۣ�11��2H2O  2H2����O2�� �� 2H2��O2
2H2����O2�� �� 2H2��O2 2H2O���𰸺������ɣ���ѧʽ����1�֣�©��������ƽ��0.5��)
2H2O���𰸺������ɣ���ѧʽ����1�֣�©��������ƽ��0.5��)
�ܣ�12���� ��13��CO2+ H2O H2CO3 ����ѧʽ����1��)
H2CO3 ����ѧʽ����1��)
�ݣ�14��10 ��15��40
��16����Ͳ���ιܡ��ձ�������������С��2�֣�ÿ����������0.5�֣������ֿ۷֣�
�ڣ�10��6.02��1024

 �ۣ�11��2H2O
�ۣ�11��2H2O  2H2����O2�� �� 2H2��O2
2H2����O2�� �� 2H2��O2 2H2O���𰸺������ɣ���ѧʽ����1�֣�©��������ƽ��0.5��)
2H2O���𰸺������ɣ���ѧʽ����1�֣�©��������ƽ��0.5��)�ܣ�12���� ��13��CO2+ H2O
 H2CO3 ����ѧʽ����1��)
H2CO3 ����ѧʽ����1��)�ݣ�14��10 ��15��40
��16����Ͳ���ιܡ��ձ�������������С��2�֣�ÿ����������0.5�֣������ֿ۷֣�
�ٷ����DZ������ʻ�ѧ���ʵ���С���ӣ�ˮ����ˮ���ӹ��ɵģ������ܱ���ˮ�Ļ�ѧ���ʵ���С������ˮ���ӣ�18gˮ�к���6.23��1023��ˮ���ӣ�90gˮ��ˮ���ӵĸ���ԼΪ��6.23��1023��5=3.1��1024��ˮ���ӣ�
�ʴ�Ϊ��ˮ���ӣ�3.1��1024��
��ˮ����������������������������Ԫ����ɣ���������Ԫ����ɣ��ڻ�ѧ��Ӧǰ��Ԫ�ص�����䣬���Կ��Ƴ�ˮ������Ԫ�غ���Ԫ����ɵģ�
�ʴ�Ϊ��
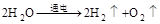 .
.�ܶ�����̼����ˮ����̼�ᣬ������ѧ��Ӧ�ķ���ʽ�ǣ�CO2+H2O�TH2CO3��̼���ʹʯ���죮������ʢ����ɫʯ����Һ���Թ���ͨ�������̼��ʯ���ɺ�ɫ��
�ʴ�Ϊ���죻CO2+H2O�TH2CO3��
����Ϊ
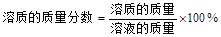 �����ԣ�
�����ԣ�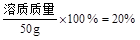 ������������10g����ˮ40mL��
������������10g����ˮ40mL��������Һ��Ҫ�õ��������У��ձ�����Ͳ������������ͷ�ιܣ�
�ʴ�Ϊ��10��40����Ͳ���ιܡ��ձ�����������

��ϰ��ϵ�д�
�����Ŀ







