��Ŀ����
�����ǵ���Ҫ�ɷ���̼��ƣ�С��ͬѧΪ�˲ⶨ��������̼��Ƶ��������������������µ�̽��ʵ�飬����������Ļ��
��ʵ����̡���������ϴ��������������������뵽��ƿ�У�����������ϡ�����ַ�Ӧ�������������ʲ������ᷴӦ����Ӧ���ɵ�����ȫ���ݳ�����
��ʵ�����ݡ�������12.5g + ϡ����40.5g  ʣ��Ļ����48.6g
ʣ��Ļ����48.6g
�����ݴ������� ���������غ㶨�ɣ����㷴Ӧ���ɶ�����̼������Ϊ ��25�� g��
�� ͨ����ѧ�� ��ʽ���㼦�����к�̼��Ƶ����ʵ���������̣� ��26��
��ʽ���㼦�����к�̼��Ƶ����ʵ���������̣� ��26��
�� ���㼦������̼��Ƶ���������������̣� ��27��
����������ʵ���У����в������ɵĶ�����̼�������ܽ���ˮ��û��ȫ���ݳ�����ɼ�������ʵ��ֵ��� ��28�� ����д��ƫ����ƫС�����䡱����

| 4.4 | ||
| 29��26�� | �輦�����к�̼��Ƶ����ʵ���x mol n��������̼��=4.4/44=0.1 mol CaCO3��2HCl��CaCl2��H2O��CO2�� 1 1 x 0.1
�𣺼������к�̼��Ƶ����ʵ���Ϊ0.1 mol | ����ʽ1�� ��ʽ1�� ���1�� |
| 29��27�� | ��������̼��Ƶ���������= | ����1�֣��ޱ���ʽ��ֻ�д𰸣������֣� |
| 29��28�� | ƫС |

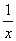 =
=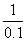 x=0.1 mol
x=0.1 mol 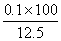 ��100% = 80%����0.8��
��100% = 80%����0.8��