��Ŀ����
��2009?��������ͼ�dz��л�ѧ���õ�ʵ��������װ�ã���������и��⣺
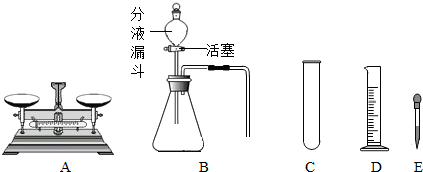
��1��ʵ����ʹ������A�ĵ�һ��������
��2����μ���װ��B��������
��3��д������C��һ����;

��1��ʵ����ʹ������A�ĵ�һ��������
������ƽƽ��
������ƽƽ��
����2����μ���װ��B��������
�ڵ������齺�ܴ��õ��ɼмн������Һ©���ڲ��ϼ�ˮ��һ��ʱ����Һ©����Һ�治���½����������ԽϺã������𰸾��ɣ�
�ڵ������齺�ܴ��õ��ɼмн������Һ©���ڲ��ϼ�ˮ��һ��ʱ����Һ©����Һ�治���½����������ԽϺã������𰸾��ɣ�
����3��д������C��һ����;
�������Լ��ķ�Ӧ�����������𰸾��ɣ�
�������Լ��ķ�Ӧ�����������𰸾��ɣ�
������E����������ͷ�ι�
��ͷ�ι�
�����������ݳ�����������;��ʹ��ע����������ش�ʹ����ƽ����Ҫ������ƽƽ�⣬���װ�õ�������ʱ�跨ʹװ�������γ���ѹ���dz����ֶΣ��Թܳ�������Ӧ�������������ʵ��ܽ�ȣ�
����⣺��1��ʵ����ʹ��������ƽ�ĵ�һ�������ǵ�����ƽƽ�⣻
�ʴ�Ϊ��������ƽƽ�⣻
��2������װ��B�������Եķ����ǣ���ס���ܣ����Һ©���ڼ���ˮ������������ˮ������ƿ��ʹƿ��ѹǿ����Һ©���ڵ�ˮ���ܼ������룬�����������ã�
�ʴ�Ϊ���ڵ������齺�ܴ��õ��ɼмн������Һ©���ڲ��ϼ�ˮ��һ��ʱ����Һ©����Һ�治���½����������ԽϺã������𰸾��ɣ���
��3���Թܳ�������Ӧ�������������ʵ��ܽ�ȣ�����E�ǽ�ͷ�ιܣ�
�ʴ�Ϊ���������Լ��ķ�Ӧ�����������𰸾��ɣ�����ͷ�ιܣ�
�ʴ�Ϊ��������ƽƽ�⣻
��2������װ��B�������Եķ����ǣ���ס���ܣ����Һ©���ڼ���ˮ������������ˮ������ƿ��ʹƿ��ѹǿ����Һ©���ڵ�ˮ���ܼ������룬�����������ã�
�ʴ�Ϊ���ڵ������齺�ܴ��õ��ɼмн������Һ©���ڲ��ϼ�ˮ��һ��ʱ����Һ©����Һ�治���½����������ԽϺã������𰸾��ɣ���
��3���Թܳ�������Ӧ�������������ʵ��ܽ�ȣ�����E�ǽ�ͷ�ιܣ�
�ʴ�Ϊ���������Լ��ķ�Ӧ�����������𰸾��ɣ�����ͷ�ιܣ�
������������Ҫ�����˳�������������;��װ�������Եļ�飬�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
 ��2009?������A��B��HΪ���嵥�ʣ�EΪ��ɫ���壬FΪ���������Ҫ�ɷ֣�KΪ��ɫ����������Һ������֮������ͼ��ʾת����ϵ��
��2009?������A��B��HΪ���嵥�ʣ�EΪ��ɫ���壬FΪ���������Ҫ�ɷ֣�KΪ��ɫ����������Һ������֮������ͼ��ʾת����ϵ��