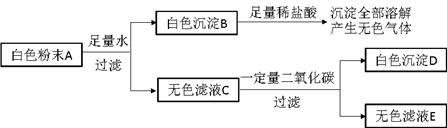
��Ŀ����
��Ԫ���������ڿ�������Һƽ�⣬ά�ּ��������˷ܺ�ϸ����ͨ�Ե����á�������Ԫ��ȫ����Դ��ʳ��(NaCl)
(1) ij������Ϊʳ�ε��������������農������һ������������ˮ(NaCl����������Ϊ0.9%���ܶ�ԼΪ1g/ml)��ҽ������ȷ��ÿ��Ӧ����NaClԼ2.7g����ÿ��������������ˮ���ٺ�����
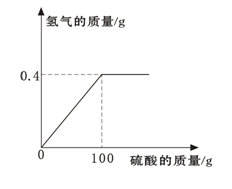
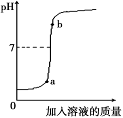
(2) ij�������Ŷ��г���һ��������ˮ��������������ȡ����������ˮ250ml����μ���ijδ֪Ũ�ȵ�AgNO3��Һ200g�����ɳ��������������AgNO3��Һ��������ϵ��ͼ��
��ͨ�������ж�����������ˮ�Ƿ������������Ϊ0.9%��ҽ�ñ���������AgNO3��Һ������������
(1) ij������Ϊʳ�ε��������������農������һ������������ˮ(NaCl����������Ϊ0.9%���ܶ�ԼΪ1g/ml)��ҽ������ȷ��ÿ��Ӧ����NaClԼ2.7g����ÿ��������������ˮ���ٺ�����
(2) ij�������Ŷ��г���һ��������ˮ��������������ȡ����������ˮ250ml����μ���ijδ֪Ũ�ȵ�AgNO3��Һ200g�����ɳ��������������AgNO3��Һ��������ϵ��ͼ��

��ͨ�������ж�����������ˮ�Ƿ������������Ϊ0.9%��ҽ�ñ���������AgNO3��Һ������������
��(1)������ˮ������= (1��)
(1��)
������ˮ����� =300ml ��1�֣�
=300ml ��1�֣�
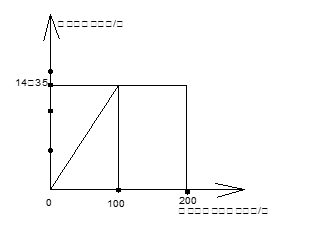
(2)��NaCl������Ϊx��AgNO3����Ϊy
NaCl + AgNO3 = AgCl��+NaNO3 (1��)
58.5 170 143.5
x y 14.35g
58.5:143.5=x:14.35g x=5.85g
170:143.5=y:14.35g y="17g" (1��)
NaCl����������=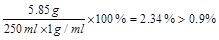 ��1�֣�
��1�֣�
����ҽ�ñ�
AgNO3����������= (1��
(1��
 (1��)
(1��)������ˮ�����
 =300ml ��1�֣�
=300ml ��1�֣�(2)��NaCl������Ϊx��AgNO3����Ϊy
NaCl + AgNO3 = AgCl��+NaNO3 (1��)
58.5 170 143.5
x y 14.35g
58.5:143.5=x:14.35g x=5.85g
170:143.5=y:14.35g y="17g" (1��)
NaCl����������=
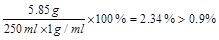 ��1�֣�
��1�֣�����ҽ�ñ�
AgNO3����������=
 (1��
(1��(1)�����Ȼ��Ƶ���������Һ�����������������������Һ���������ٸ�����Һ���ܶȼ������Һ�����
(2)�Ȼ��ƿ��Ժ���������Ӧ�����Ȼ�����������Ŀ���Ѹ������Ȼ������������Կ��Ծݴ�����Ȼ��Ƶ�������Ȼ�����������ˮ��������ܶ������ܶȹ�ʽ�������������ˮ���������������������ˮ��������������0.9%��ȽϺ��ж��Ƿ����ҽ�ñ���
(2)�Ȼ��ƿ��Ժ���������Ӧ�����Ȼ�����������Ŀ���Ѹ������Ȼ������������Կ��Ծݴ�����Ȼ��Ƶ�������Ȼ�����������ˮ��������ܶ������ܶȹ�ʽ�������������ˮ���������������������ˮ��������������0.9%��ȽϺ��ж��Ƿ����ҽ�ñ���

��ϰ��ϵ�д�
�����Ŀ

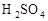
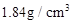 ����������98%
����������98%