��Ŀ����
13��������60��ı���NaCl��Һ�������Һ�йص����У���ˮ������������Һ������NaCl����������NaCl��������������60��ʱNaCl���ܽ�ȣ�����Һ���������������գ�
��1�����ñ�����Һϡ�ͣ���������� �ڢܣ�
��2�����ñ�����Һ������20�棬һ���仯������ �ڢۢݣ�
���H��C��O��Na����Ԫ���У�ѡ���ʵ���Ԫ����ɷ�������Ҫ������ʣ����û�ѧʽ��գ�
��1���׳ơ�С�մ������� NaHCO3��
��2���ҹ���������ѧ�Һ�°��о��ɹ��ġ������Ƽ���������ġ���� Na2CO3��
��1�����ñ�����Һϡ�ͣ���������� �ڢܣ�
��2�����ñ�����Һ������20�棬һ���仯������ �ڢۢݣ�
���H��C��O��Na����Ԫ���У�ѡ���ʵ���Ԫ����ɷ�������Ҫ������ʣ����û�ѧʽ��գ�
��1���׳ơ�С�մ������� NaHCO3��
��2���ҹ���������ѧ�Һ�°��о��ɹ��ġ������Ƽ���������ġ���� Na2CO3��
����������ݱ�����Һ�ĸ��һ���¶��£�һ�����ܼ�����ܼ����ܽ�ij���ʵ���Һ�������ʽ�������
�����С�մ�ʹ���ijɷֽ��з�����
�����С�մ�ʹ���ijɷֽ��з�����
����𣺢壨1����60��ı���NaCl��Һ����ˮϡ�ͺ���Ϊ�¼ӽ���ˮ������ˮ�����������Ӣ���Ϊֻ�������ܼ���������Һ������NaCl�������������Ϊ��ˮϡ���ˣ�����NaCl����������Ҫ��С����ij�¶��£�ij���ʵ��ܽ����һ������������������������ض��ı䣬����60��ʱNaCl���ܽ�Ȳ������Ϊ�ܼ����������ˣ�������Һ������Ҳ�����ӣ�
�ʴ�Ϊ���ڢ�
��2�����ñ�����Һ������20�����Ϊû�иı�ˮ������������ˮ�������������Ϊ�Ȼ��Ƶ��ܽ�����¶ȵĽ��Ͷ����ͣ����Խ����¶Ⱥ�Ҫ��һ����������������������Һ������NaCl������Ҫ��С������Ϊ�Ȼ��Ƶ��ܽ�����¶ȵĽ��Ͷ����ͣ����Խ����¶Ⱥ�NaCl����������Ҫ���ͣ���ij�¶��£�ij���ʵ��ܽ����һ������������������������ض��ı䣬����60��ʱNaCl���ܽ�Ȳ��䣻����Ϊ�Ȼ��Ƶ��ܽ�����¶ȵĽ��Ͷ����ͣ����Խ����¶Ⱥ�һ�������ʻ���������������Һ������Ҫ��С��
�ʴ�Ϊ���ڢۢ�
��С�մ����Ҫ�ɷ���̼�����ƣ��������Ƽ���еļ�ָ���Ǵ������̼���ƣ�
�ʴ�Ϊ����1�� NaHCO3 ��2�� Na2CO3
�ʴ�Ϊ���ڢ�
��2�����ñ�����Һ������20�����Ϊû�иı�ˮ������������ˮ�������������Ϊ�Ȼ��Ƶ��ܽ�����¶ȵĽ��Ͷ����ͣ����Խ����¶Ⱥ�Ҫ��һ����������������������Һ������NaCl������Ҫ��С������Ϊ�Ȼ��Ƶ��ܽ�����¶ȵĽ��Ͷ����ͣ����Խ����¶Ⱥ�NaCl����������Ҫ���ͣ���ij�¶��£�ij���ʵ��ܽ����һ������������������������ض��ı䣬����60��ʱNaCl���ܽ�Ȳ��䣻����Ϊ�Ȼ��Ƶ��ܽ�����¶ȵĽ��Ͷ����ͣ����Խ����¶Ⱥ�һ�������ʻ���������������Һ������Ҫ��С��
�ʴ�Ϊ���ڢۢ�
��С�մ����Ҫ�ɷ���̼�����ƣ��������Ƽ���еļ�ָ���Ǵ������̼���ƣ�
�ʴ�Ϊ����1�� NaHCO3 ��2�� Na2CO3
������������Һ������˵��Զ�DZ�����Һ����������������������ĸı���ı䣬�������ʡ��ܼ�����Һ������Ҳ����һ���ĸı䣮

��ϰ��ϵ�д�
 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
�����Ŀ
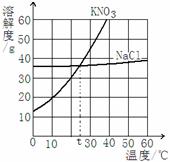 24����1������60��ı���NaCl��Һ�������Һ�йص����У���ˮ������������Һ������NaCl����������NaCl��������������60��ʱNaCl���ܽ�ȣ�����Һ���������������գ�
24����1������60��ı���NaCl��Һ�������Һ�йص����У���ˮ������������Һ������NaCl����������NaCl��������������60��ʱNaCl���ܽ�ȣ�����Һ���������������գ�
 ��1������60��ı���NaCl��Һ�������Һ�йص����У���ˮ������������Һ������NaCl����������NaCl��������������60��ʱNaCl���ܽ�ȣ�����Һ���������������գ�
��1������60��ı���NaCl��Һ�������Һ�йص����У���ˮ������������Һ������NaCl����������NaCl��������������60��ʱNaCl���ܽ�ȣ�����Һ���������������գ�
