��Ŀ����
��8�֣���1������60��ı���NaCl��Һ,�����Һ�йص�����:��ˮ������;����Һ������NaCl������;��NaCl����������;��60��ʱNaCl���ܽ��:����Һ����������������:
�ٽ��ñ�����Һϡ��,���������: ;
�ڽ��ñ�����Һ������20��,һ���仯������ ��
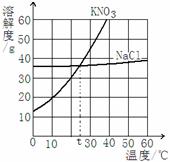
��2������KNO3��NaCl���ܽ�����ߣ��ش��������⣺
��10��ʱKNO3���ܽ�� 60��ʱNaCl�ܽ��(�����������������)��
��t��ʱ����mg KNO3��ng NaCl �ֱ��ܽ���20mLˮ��ǡ�ñ��ͣ���m n(�����������������)��
��8�֣�(1)�ڢ� �ڢۢ� (2) < =
����

��ϰ��ϵ�д�
 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
�����Ŀ
 24����1������60��ı���NaCl��Һ�������Һ�йص����У���ˮ������������Һ������NaCl����������NaCl��������������60��ʱNaCl���ܽ�ȣ�����Һ���������������գ�
24����1������60��ı���NaCl��Һ�������Һ�йص����У���ˮ������������Һ������NaCl����������NaCl��������������60��ʱNaCl���ܽ�ȣ�����Һ���������������գ�
 ��1������60��ı���NaCl��Һ�������Һ�йص����У���ˮ������������Һ������NaCl����������NaCl��������������60��ʱNaCl���ܽ�ȣ�����Һ���������������գ�
��1������60��ı���NaCl��Һ�������Һ�йص����У���ˮ������������Һ������NaCl����������NaCl��������������60��ʱNaCl���ܽ�ȣ�����Һ���������������գ�
