��Ŀ����
�����Ķ�ӰҺ�к���һ������AgNO3��ij����С�����Ӱ���ռ���һЩ�����Ķ�ӰҺ���������е����Ե�����ʽȫ�����ա�������������µ�ʵ�鷽����
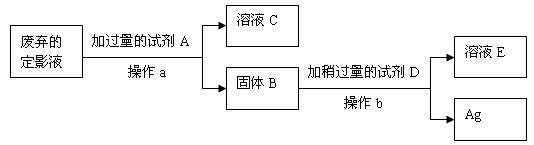
�ʣ�
��1��ͬѧ������Լ�Aѡ�����ۣ���ʱ�Լ�DӦ��ѡ��_______��ͬѧ������Լ�Aѡ��ͭ�ۣ���ʱ�Լ�DӦ��ѡ��__________���������ַ����У�����Ϊͬѧ_________������ң��ķ����Ϻ�����������___________________________��
��2������a��___________����Ҫ�õ��IJ����������ձ�����������___________��
��1��ͬѧ������Լ�Aѡ�����ۣ���ʱ�Լ�DӦ��ѡ��_______��ͬѧ������Լ�Aѡ��ͭ�ۣ���ʱ�Լ�DӦ��ѡ��__________���������ַ����У�����Ϊͬѧ_________������ң��ķ����Ϻ�����������___________________________��
��2������a��___________����Ҫ�õ��IJ����������ձ�����������___________��
��1��ϡ���ᣨ��ϡ���ᣩ����������Һ���ף���������ͭ�����ã�������ϡ���ᣨ��ϡ���ᣩ��ȥ����������Ҳ�Ƚϼ��;��ã��ؼ����������ҷ��������ã���
��2�����ˣ�©��
��2�����ˣ�©��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
