��Ŀ����
 ����μӵĻ�ѧʵ�鿼�����Ŀ�ǡ���εļ��顱���Իش���������
����μӵĻ�ѧʵ�鿼�����Ŀ�ǡ���εļ��顱���Իش�����������1��������ƷΪ�Ȼ�泥���д��ʵ������з�����Ӧ�Ļ�ѧ����ʽ
NH4Cl+NaOH
NaCl+H2O+NH3��
| ||
NH4Cl+NaOH
NaCl+H2O+NH3��
��
| ||
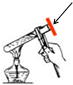
��2����ͼΪ��ޱͬѧ����笠����ӵIJ���ͼ����ָ�����������ֵ�ԭ��
| ��� | �۷�ԭ�� |
| 1 | �Թ���Һ��̫�� �Թ���Һ��̫�� |
| 2 | ����ʱ��Ĵָ�����ԹܼеĶ̱� ����ʱ��Ĵָ�����ԹܼеĶ̱� |
ˮ��������ֽʪ��
ˮ��������ֽʪ��
��4������Ϊ����ʵ������ʱû���ҵ���ɫʯ����ֽ��������ʱ������Ʒ��
pH��ֽ
pH��ֽ
����������1��������εļ����ϻ�ѧ����ʽ����д������
��2�����ݸ��Թ���Һ����ȵ�ע��������з�����
��3������ˮ��������ֽʪ��Ӷ����б�ɫ���з�����
��4��pH��ֽ���������ɫʯ����ֽ��
��2�����ݸ��Թ���Һ����ȵ�ע��������з�����
��3������ˮ��������ֽʪ��Ӷ����б�ɫ���з�����
��4��pH��ֽ���������ɫʯ����ֽ��
����⣺��1����εļ��飺����Ʒ�����Թܼ��ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�ʪ���ɫʯ����ֽ�����ɫ��֤�������������ǰ�����֤�������к���笠����ӣ��йط�Ӧ�Ļ�ѧ����ʽ��NH4Cl+NaOH
NaCl+H2O+NH3�����ʴ�Ϊ��NH4Cl+NaOH
NaCl+H2O+NH3����
��2��ͼ�д������Թ���Һ��̫�࣬����ʱ��Ĵָ�����ԹܼеĶ̱����ʴ�Ϊ���Թ���Һ��̫�ࣻ����ʱ��Ĵָ�����ԹܼеĶ̱���
��3�����Թ��ڵ�Һ��������һ�����ˮ��������ֽʪ�������ֽҲ�����ɫ���ʴ�Ϊ��ˮ��������ֽʪ��
��4��pH��ֽ���������ɫʯ����ֽ���ʴ�Ϊ��pH��ֽ��
| ||
| ||
��2��ͼ�д������Թ���Һ��̫�࣬����ʱ��Ĵָ�����ԹܼеĶ̱����ʴ�Ϊ���Թ���Һ��̫�ࣻ����ʱ��Ĵָ�����ԹܼеĶ̱���
��3�����Թ��ڵ�Һ��������һ�����ˮ��������ֽʪ�������ֽҲ�����ɫ���ʴ�Ϊ��ˮ��������ֽʪ��
��4��pH��ֽ���������ɫʯ����ֽ���ʴ�Ϊ��pH��ֽ��
�����������ѶȽϴ�����笠����ӵļ��鷽���Լ�ע�����ʵ�����ע��������ǽ���Ĺؼ���

��ϰ��ϵ�д�
 �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�
�����Ŀ
 ����μӵĻ�ѧʵ�鿼�����Ŀ�ǡ���εļ��顱���Իش���������
����μӵĻ�ѧʵ�鿼�����Ŀ�ǡ���εļ��顱���Իش���������
��1��������ƷΪ�Ȼ�泥���д��ʵ������з�����Ӧ�Ļ�ѧ����ʽ______��
��2����ͼΪ��ޱͬѧ����笠����ӵIJ���ͼ����ָ�����������ֵ�ԭ��
| ��� | �۷�ԭ�� |
| 1 | ______ |
| 2 | ______ |
��4������Ϊ����ʵ������ʱû���ҵ���ɫʯ����ֽ��������ʱ������Ʒ��______��
 �������г��л�ѧʵ�鿼��������ǡ�ʵ������ȡ������̼���塱����ش�����������⣮
�������г��л�ѧʵ�鿼��������ǡ�ʵ������ȡ������̼���塱����ش�����������⣮

 A������������Լ����֮һ�����Թܿ�
A������������Լ����֮һ�����Թܿ� Ӧ���ɵ�̼���ֺܲ��ȶ���������ˮ���ռ�CO2
Ӧ���ɵ�̼���ֺܲ��ȶ���������ˮ���ռ�CO2 (3)��ͼ2��ʾ������CO2���Թ��ڵ���Լռ�Թ��ݻ�����֮һ�ij���ʯ��ˮ��
(3)��ͼ2��ʾ������CO2���Թ��ڵ���Լռ�Թ��ݻ�����֮һ�ij���ʯ��ˮ��