��Ŀ����
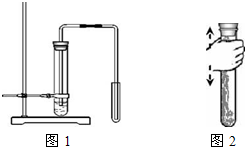 �������г��л�ѧʵ�鿼��������ǡ�ʵ������ȡ������̼���塱����ش�����������⣮
�������г��л�ѧʵ�鿼��������ǡ�ʵ������ȡ������̼���塱����ش�����������⣮��1�������й��������������������У�������ǣ�
C
C
������ţ���A������������Լ����֮һ�����Թܿ�
B���齺���벣����������ǰ��ˮ��ʪ�ܿ�
C���齺��һ����������������
D���������ܿ�һ�������齺��һ��
��2����ͼ1��ij����Ƶ��Ʊ�������̼��ʾ��ͼ��������
3
3
��������д��ʵ�����Ʊ�������̼����ķ��ű���ʽ
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
����ʵ�����������ſ������ռ�CO2����ʱ��ν�������
��ȼ�ŵ�ľ���ŵ�����ƿ�ڣ����ľ��Ϩ��֤��������̼���ռ���
��ȼ�ŵ�ľ���ŵ�����ƿ�ڣ����ľ��Ϩ��֤��������̼���ռ���
����3����ͼ2��ʾ������CO2���Թ��ڵ���Լռ�Թ��ݻ�����֮һ�ij���ʯ��ˮ�������������ϡ��³�����һ��ʱ�䣮
��д��ʵ���Ҽ���CO2�ķ��ű���ʽ
CO2+Ca��OH��2�TCaCO3��+H2O
CO2+Ca��OH��2�TCaCO3��+H2O
���ڳ�����Թ���ѹǿ
��
��
�Թ������ѹǿ��ѡ�������=���������������Թ�ǰ������������Ŀ���ǣ�
��ֹʯ��ˮ����Թܸ�ʴƤ��
��ֹʯ��ˮ����Թܸ�ʴƤ��
���ٽ�ʯ��ˮ�������CO2
�ٽ�ʯ��ˮ�������CO2
����������1���й��������������������У�������ǣ��齺��һ���������������ڣ�Ӧ���Dz������ܿ�һ�������齺��һ�ˣ��齺���벣����������ǰ��ˮʪ��ܿڣ�Ӧ���Dz������ܿ���ˮʪ��
��2��ͼ1��ij����Ƶ��Ʊ�������̼��ʾ��ͼ�����еĴ����У�����������Ƥ��̫���ˣ�����Һ���ڣ�����������������Ӧ�ü����Թܵ����ϲ����ռ�������Թ�û�й̶�����ȡװ�ð������ȺͲ���������֣�ʵ������ȡCO2�����ڳ����£���̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����˲���Ҫ���ȣ�������̼������ˮ���ܶȱȿ������ܶȴ����ֻ���������ſ������ռ���������̼��ȼ��Ҳ��֧��ȼ�գ����Կ���ȼ�ŵ�ľ�����������̼�Ƿ��ռ�����
��3��������̼һ���ó���ʯ��ˮ���飬������̼���������Ʒ�Ӧ����̼��ư�ɫ������ˮ��������Թ���ѹǿС���Թ��ڴ���ѹǿ����Ϊ������̼��Ӧ�ˣ����Թ�ǰ������������Ŀ���ǣ���ֹʯ��ˮ����Թܸ�ʴƤ�����ٽ�ʯ��ˮ�������CO2��
��2��ͼ1��ij����Ƶ��Ʊ�������̼��ʾ��ͼ�����еĴ����У�����������Ƥ��̫���ˣ�����Һ���ڣ�����������������Ӧ�ü����Թܵ����ϲ����ռ�������Թ�û�й̶�����ȡװ�ð������ȺͲ���������֣�ʵ������ȡCO2�����ڳ����£���̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����˲���Ҫ���ȣ�������̼������ˮ���ܶȱȿ������ܶȴ����ֻ���������ſ������ռ���������̼��ȼ��Ҳ��֧��ȼ�գ����Կ���ȼ�ŵ�ľ�����������̼�Ƿ��ռ�����
��3��������̼һ���ó���ʯ��ˮ���飬������̼���������Ʒ�Ӧ����̼��ư�ɫ������ˮ��������Թ���ѹǿС���Թ��ڴ���ѹǿ����Ϊ������̼��Ӧ�ˣ����Թ�ǰ������������Ŀ���ǣ���ֹʯ��ˮ����Թܸ�ʴƤ�����ٽ�ʯ��ˮ�������CO2��
����⣺��1���й��������������������У�������ǣ��齺��һ���������������ڣ�Ӧ���Dz������ܿ�һ�������齺��һ�ˣ��齺���벣����������ǰ��ˮʪ��ܿڣ�Ӧ���Dz������ܿ���ˮʪ�ʴ�Ϊ��C
��2����ͼ1��ij����Ƶ��Ʊ�������̼��ʾ��ͼ�����еĴ����У�����������Ƥ��̫���ˣ�����Һ���ڣ�����������������Ӧ�ü����Թܵ����ϲ����ռ�������Թ�û�й̶���
����̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����ƽ���ɣ����ű���ʽΪCaCO3+2HCl�TCaCl2+H2O+CO2����
�۶�����̼��ȼ��Ҳ��֧��ȼ�գ����Կ���ȼ�ŵ�ľ�����������̼�Ƿ��ռ�������ȼ�ŵ�ľ���ŵ�����ƿ�ڣ����ľ��Ϩ��֤��������̼���ռ�����
���ʴ�Ϊ����3����CaCO3+2HCl�TCaCl2+H2O+CO2�����۽�ȼ�ŵ�ľ���ŵ�����ƿ�ڣ����ľ��Ϩ��֤��������̼���ռ�����
��3���ٶ�����̼һ���ó���ʯ��ˮ���飬������̼���������Ʒ�Ӧ����̼��ư�ɫ������ˮ�����ű���ʽΪCO2+Ca��OH��2�TCaCO3��+H2O��
�ڳ�����Թ���ѹǿС�ڴ���ѹǿ����Ϊ������̼��Ӧ�ˣ�
�����Թ�ǰ������������Ŀ���ǣ���ֹʯ��ˮ����Թܸ�ʴƤ�����ٽ�ʯ��ˮ�������CO2��
�ʴ�Ϊ����CO2+Ca��OH��2�TCaCO3��+H2O���ڣ����۷�ֹʯ��ˮ����Թܸ�ʴƤ�����ٽ�ʯ��ˮ�������CO2��
��2����ͼ1��ij����Ƶ��Ʊ�������̼��ʾ��ͼ�����еĴ����У�����������Ƥ��̫���ˣ�����Һ���ڣ�����������������Ӧ�ü����Թܵ����ϲ����ռ�������Թ�û�й̶���
����̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����ƽ���ɣ����ű���ʽΪCaCO3+2HCl�TCaCl2+H2O+CO2����
�۶�����̼��ȼ��Ҳ��֧��ȼ�գ����Կ���ȼ�ŵ�ľ�����������̼�Ƿ��ռ�������ȼ�ŵ�ľ���ŵ�����ƿ�ڣ����ľ��Ϩ��֤��������̼���ռ�����
���ʴ�Ϊ����3����CaCO3+2HCl�TCaCl2+H2O+CO2�����۽�ȼ�ŵ�ľ���ŵ�����ƿ�ڣ����ľ��Ϩ��֤��������̼���ռ�����
��3���ٶ�����̼һ���ó���ʯ��ˮ���飬������̼���������Ʒ�Ӧ����̼��ư�ɫ������ˮ�����ű���ʽΪCO2+Ca��OH��2�TCaCO3��+H2O��
�ڳ�����Թ���ѹǿС�ڴ���ѹǿ����Ϊ������̼��Ӧ�ˣ�
�����Թ�ǰ������������Ŀ���ǣ���ֹʯ��ˮ����Թܸ�ʴƤ�����ٽ�ʯ��ˮ�������CO2��
�ʴ�Ϊ����CO2+Ca��OH��2�TCaCO3��+H2O���ڣ����۷�ֹʯ��ˮ����Թܸ�ʴƤ�����ٽ�ʯ��ˮ�������CO2��
��������������Ҫ���������������ӡ��������ȡװ�ú��ռ�װ�õ�ѡ��ͬʱҲ�����˻�ѧ����ʽ����д���ۺ��ԱȽ�ǿ���������ȡװ�õ�ѡ���뷴Ӧ���״̬�ͷ�Ӧ�������йأ�������ռ�װ�õ�ѡ����������ܶȺ��ܽ����йأ����������п�����Ҫ����֮һ����Ҫ������ʵ�����У�

��ϰ��ϵ�д�
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
�����Ŀ
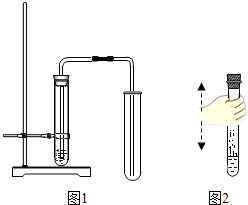 ��2013?���ݣ��������г��л�ѧʵ�鿼��������ǡ�ʵ������ȡ������̼���塱����ش�����������⣮
��2013?���ݣ��������г��л�ѧʵ�鿼��������ǡ�ʵ������ȡ������̼���塱����ش�����������⣮

 A������������Լ����֮һ�����Թܿ�
A������������Լ����֮һ�����Թܿ� Ӧ���ɵ�̼���ֺܲ��ȶ���������ˮ���ռ�CO2
Ӧ���ɵ�̼���ֺܲ��ȶ���������ˮ���ռ�CO2 (3)��ͼ2��ʾ������CO2���Թ��ڵ���Լռ�Թ��ݻ�����֮һ�ij���ʯ��ˮ��
(3)��ͼ2��ʾ������CO2���Թ��ڵ���Լռ�Թ��ݻ�����֮һ�ij���ʯ��ˮ��