��Ŀ����
������һЩ���õ�����ͼ����ش�����
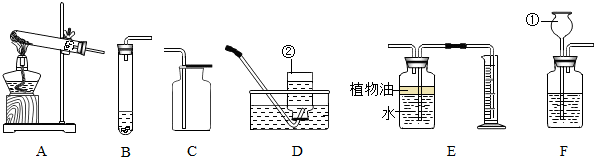
��1����ͼA��B��C��D����ֱ�Ӽ��ȵ�������
��2����Ҫ������������װCO2��ȡװ�ã�������Ҫѡ�õ�������
��3����Ҫѡ������C��D��F��G��H���һ���ø��������ȡ������װ�ã����������ͼ��ѡ�õ�������

��1����ͼA��B��C��D����ֱ�Ӽ��ȵ�������
�Թ�
�Թ�
����д�������ƣ�����2����Ҫ������������װCO2��ȡװ�ã�������Ҫѡ�õ�������
AEJ
AEJ
������ţ���ʵ������ȡ������̼�Ļ�ѧ����ʽΪCaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
��װҩƷǰ�����������ȡװ�ý��еIJ��������װ�õ�������
���װ�õ�������
����3����Ҫѡ������C��D��F��G��H���һ���ø��������ȡ������װ�ã����������ͼ��ѡ�õ�������
����̨
����̨
����д�������ƣ���һ�ֱر���ʵ����Ʒ����
��
���ø��������ȡ�����Ļ�ѧ����ʽΪ2KMnO4
K2MnO4+MnO2+O2��
| ||
2KMnO4
K2MnO4+MnO2+O2��
��
| ||
��������1������ʵ���п�ֱ֪�Ӽ��ȵ������У��Թܡ�������������ȼ�ճ�D��������ȷ���жϣ�
��2��������ȡ������̼�ķ�Ӧ��״̬ȷ������ʵ������������ʵ������ȡ������̼�ķ�Ӧԭ��������������ȡ����Ļ������迼�ǣ�
��3�����ݸ��������ȡ���ռ�������װ��ͼ�ش�ѡ���������ʵ����Ʒ������д����ʽ�IJ���д������ʽ��
��2��������ȡ������̼�ķ�Ӧ��״̬ȷ������ʵ������������ʵ������ȡ������̼�ķ�Ӧԭ��������������ȡ����Ļ������迼�ǣ�
��3�����ݸ��������ȡ���ռ�������װ��ͼ�ش�ѡ���������ʵ����Ʒ������д����ʽ�IJ���д������ʽ��
����⣺��1��ʵ���п�ֱ�Ӽ��ȵ������У��Թܡ�������������ȼ�ճȣ�ͼ��A��B��C��D��ֻ���Թܿ���ֱ�Ӽ��ȣ��ʴ�Ϊ���Թܣ�
��2��������CO2�ķ�Ӧ����̼��ƺ�ϡ���ᣬ����Ҫ���ȣ�����ƿ��˫����������©�����ɣ�ʵ������ȡ������̼�ķ�Ӧ����̼��ƺ����ᣬ���������Ȼ��ơ�ˮ��������̼���ù۲취��ƽ���ɣ���ȡ����ǰ������װ�õ������ԣ����������©�����ռ��������壬�ʴ�Ϊ��AEJ��CaCO3+2HCl�TCaCl2+H2O+CO2�������װ�õ������ԣ�
��3��������������������˸����������⣬��Ϊ��Ҫ������̨�̶�����˻�������̨��ʵ�����Է�������ط�ĩ���뵼�ܣ��������Թܿ���һ�������ø�������������Ļ�ѧ����ʽ��2KMnO4
K2MnO4+MnO2+O2�����ʴ�Ϊ������̨������2KMnO4
K2MnO4+MnO2+O2����
��2��������CO2�ķ�Ӧ����̼��ƺ�ϡ���ᣬ����Ҫ���ȣ�����ƿ��˫����������©�����ɣ�ʵ������ȡ������̼�ķ�Ӧ����̼��ƺ����ᣬ���������Ȼ��ơ�ˮ��������̼���ù۲취��ƽ���ɣ���ȡ����ǰ������װ�õ������ԣ����������©�����ռ��������壬�ʴ�Ϊ��AEJ��CaCO3+2HCl�TCaCl2+H2O+CO2�������װ�õ������ԣ�
��3��������������������˸����������⣬��Ϊ��Ҫ������̨�̶�����˻�������̨��ʵ�����Է�������ط�ĩ���뵼�ܣ��������Թܿ���һ�������ø�������������Ļ�ѧ����ʽ��2KMnO4
| ||
| ||
���������⿼��ʵ��������ѡ��ѧ����ʽ����д����ѧʵ���е�������⣮����ͬѧ�ǶԻ���֪ʶ�����գ�

��ϰ��ϵ�д�
�����Ŀ