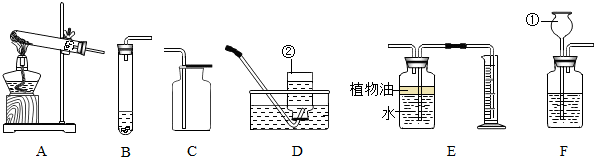
��Ŀ����
������ʵ���ҳ��õ�һЩʵ��װ�ã���ش��������⣺

��1��д����������������ƣ���
��2����ʵ�����ø��������ȡ������Ӧѡ�õ���ȡװ����
��3����ѡ��B��C�����ʵ���ҿ���ȡCO2���壬��Ӧ����ʽΪ
��4��Eװ�ÿ�������������CO2����������������ˮ���Ϸ�һ��ֲ���͵�Ŀ����
��5��Ϊ̽��������̼�����ʣ���������ʵ�飮
�ٽ�������̼ͨ��ˮ�У���pH��ֽ���Բⶨ������̼ˮ��Һ�������ǿ����������ⶨ�ķ�����
�ڽ�������̼ͨ������������Һ�У��������Է�Ӧ�������û�ѧ����֤��C02��NaOH��Һȷʵ�����˻�ѧ��Ӧ�����NaCl��Һ��CaCl2��Һ��ϡ���������Լ���ѡ��һ���Լ�����ʵ�飬����֤�������ɣ�
�۽�����C02����ͨ��ʢ�д�������Ca��OH��2��Һ�Ĵ��ձ��У��ձ�����Һ��������֮ǰ��Ȼ�
A������ B������ c������ D�����ж�
��6��ȡ12gʯ��ʯ����Ҫ�ɷ���CaC03�����ʲ��μӷ�Ӧ�������ձ��У������м���100gһ������������ϡ���ᣬ����ǡ����ȫ��Ӧ����Ӧ���������ձ���ʣ�����ʵ�������Ϊ107.6g���������ձ����������������ܽ���Բ��ƣ�����ϡ���������ʵ����������Ƕ��٣�

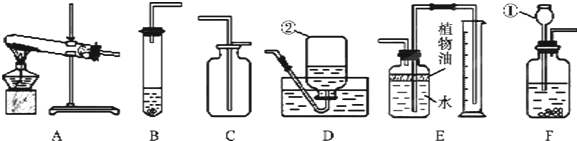
��1��д����������������ƣ���
����©��
����©��
��������ƿ
����ƿ
����2����ʵ�����ø��������ȡ������Ӧѡ�õ���ȡװ����
A
A
������ţ���ͬ������Ӧ�Ļ�ѧ����ʽ��2KMn04
K2Mn04+Mn02+02��
| ||
2KMn04
K2Mn04+Mn02+02��
��
| ||
��3����ѡ��B��C�����ʵ���ҿ���ȡCO2���壬��Ӧ����ʽΪ
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
����4��Eװ�ÿ�������������CO2����������������ˮ���Ϸ�һ��ֲ���͵�Ŀ����
ֲ���Ϳɱ���CO2��ˮ�Ӵ������ܽ⣬���²�����
ֲ���Ϳɱ���CO2��ˮ�Ӵ������ܽ⣬���²�����
����5��Ϊ̽��������̼�����ʣ���������ʵ�飮
�ٽ�������̼ͨ��ˮ�У���pH��ֽ���Բⶨ������̼ˮ��Һ�������ǿ����������ⶨ�ķ�����
�ò�����պȡ��Һ�ε�pH��ֽ�ϣ���ɫ�������ɫ�����գ�������ֵ
�ò�����պȡ��Һ�ε�pH��ֽ�ϣ���ɫ�������ɫ�����գ�������ֵ
���ڽ�������̼ͨ������������Һ�У��������Է�Ӧ�������û�ѧ����֤��C02��NaOH��Һȷʵ�����˻�ѧ��Ӧ�����NaCl��Һ��CaCl2��Һ��ϡ���������Լ���ѡ��һ���Լ�����ʵ�飬����֤�������ɣ�
ȡ������Ӧ�����Һ������CaCl2��Һ��������ɫ������֤��CO2��NaOH��Һȷʵ�����˻�ѧ��Ӧ
ȡ������Ӧ�����Һ������CaCl2��Һ��������ɫ������֤��CO2��NaOH��Һȷʵ�����˻�ѧ��Ӧ
���۽�����C02����ͨ��ʢ�д�������Ca��OH��2��Һ�Ĵ��ձ��У��ձ�����Һ��������֮ǰ��Ȼ�
B
B
����д���и�����ţ���A������ B������ c������ D�����ж�
��6��ȡ12gʯ��ʯ����Ҫ�ɷ���CaC03�����ʲ��μӷ�Ӧ�������ձ��У������м���100gһ������������ϡ���ᣬ����ǡ����ȫ��Ӧ����Ӧ���������ձ���ʣ�����ʵ�������Ϊ107.6g���������ձ����������������ܽ���Բ��ƣ�����ϡ���������ʵ����������Ƕ��٣�
��������1����dz������������ƺ���;����2�����ݷ�Ӧ���״̬ȷ������װ�ã����ݷ���ʽ����д����д����ȡ�����ķ�Ӧԭ������3�����ݷ���ʽ����д���DZ��⣻��4�����ݶ�����̼���ܽ��Կ��ǣ���5���ٸ���pHֵ�IJⶨ�������ǣ��ڸ��ݶ�����̼���������Ʒ�Ӧ�������������֤��������ڵķ������۸��ݷ�Ӧ������������Һ�����ı仯����6�������ձ������������ļ����������ɶ�����̼���������ٸ��ݶ�����̼������������������Ȼ�����������ٳ���������������ɣ�
����⣺��1���پ��Ƚϳ���©����û�п��أ����ڳ���©�������ռ�����������Ǽ���ƿ��
��2����ȡ����ķ�Ӧ���״̬�ǹ����Һ��ʱ������Ҫ���ȣ������������巴Ӧ��ȡ������Ҫ���ȣ�����������������ڹ�����Ҫ���ȣ���Ҫ�ƾ��ƣ�д����ʽҪע��һд������ע���ĵȺţ��ø��������������ƽ�������ù۲취��ƽ����Ӧ���Ǹ�����أ�������������ء��������̺�������������������������ţ�
��3��ʵ������ȡCO2�ķ�Ӧ����̼�����ϡ���ᣬ���������Ȼ��ơ�ˮ��������̼��ͨ���۲취��ƽ���ɣ�������̼��������������ţ�
��4�����ڶ�����̼������ˮ������ֱ����ˮ�Ӵ����ܽˮ�У�Ӱ��ʵ������������ֲ���Ϳɱ���CO2��ˮ�Ӵ������ܽ⣬���²�����
��5���ٲ�pHֵ���巽���ǣ��ò�����պȡ������Һ�ε�pH��ֽ�ϣ���ɫ�������ɫ�����գ�������ֵ��
�����ڶ�����̼���������Ʒ�Ӧ������̼���ƺ�ˮ��ֻҪ����֤��̼������Ӵ��ڼ��ɣ�ȡ������Ӧ�����Һ������CaCl2��Һ�����������ɫ��������̼��Ƴ�����֤��CO2��NaOH��Һȷʵ�����˻�ѧ��Ӧ��
�����ڶ�����̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ����Ȼ�ж�����̼������Һ�������ɵ�̼������ڳ�������������Һ�������ձ�����Һ��������֮ǰ��Ȼ���٣�
��6���⣺���ɶ�����̼������Ϊ��12g+100g-107.6g=4.4g
��ϡ�������Ȼ��������Ϊ x��
CaCO3+2HCl�TCaCl2+H2O+CO2��
73 44
x 4.4g
���ݣ�
=
���x=7.3g��
ϡ���������ʵ�����������
��100%=7.3%
��ϡ���������ʵ���������Ϊ7.3%
�ʴ�Ϊ����1������©��������ƿ����2��A�� 2KMn04
K2Mn04+Mn02+02������3��CaCO3+2HCl�TCaCl2+H2O+CO2������4��ֲ���Ϳɱ���CO2��ˮ�Ӵ������ܽ⣬���²�������5�����ò�����պȡ��Һ�ε�pH��ֽ�ϣ���ɫ�������ɫ�����գ�������ֵ����ȡ������Ӧ�����Һ������CaCl2��Һ��������ɫ������֤��CO2��NaOH��Һȷʵ�����˻�ѧ��Ӧ����B��6��7.3%��
��2����ȡ����ķ�Ӧ���״̬�ǹ����Һ��ʱ������Ҫ���ȣ������������巴Ӧ��ȡ������Ҫ���ȣ�����������������ڹ�����Ҫ���ȣ���Ҫ�ƾ��ƣ�д����ʽҪע��һд������ע���ĵȺţ��ø��������������ƽ�������ù۲취��ƽ����Ӧ���Ǹ�����أ�������������ء��������̺�������������������������ţ�
��3��ʵ������ȡCO2�ķ�Ӧ����̼�����ϡ���ᣬ���������Ȼ��ơ�ˮ��������̼��ͨ���۲취��ƽ���ɣ�������̼��������������ţ�
��4�����ڶ�����̼������ˮ������ֱ����ˮ�Ӵ����ܽˮ�У�Ӱ��ʵ������������ֲ���Ϳɱ���CO2��ˮ�Ӵ������ܽ⣬���²�����
��5���ٲ�pHֵ���巽���ǣ��ò�����պȡ������Һ�ε�pH��ֽ�ϣ���ɫ�������ɫ�����գ�������ֵ��
�����ڶ�����̼���������Ʒ�Ӧ������̼���ƺ�ˮ��ֻҪ����֤��̼������Ӵ��ڼ��ɣ�ȡ������Ӧ�����Һ������CaCl2��Һ�����������ɫ��������̼��Ƴ�����֤��CO2��NaOH��Һȷʵ�����˻�ѧ��Ӧ��
�����ڶ�����̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ����Ȼ�ж�����̼������Һ�������ɵ�̼������ڳ�������������Һ�������ձ�����Һ��������֮ǰ��Ȼ���٣�
��6���⣺���ɶ�����̼������Ϊ��12g+100g-107.6g=4.4g
��ϡ�������Ȼ��������Ϊ x��
CaCO3+2HCl�TCaCl2+H2O+CO2��
73 44
x 4.4g
���ݣ�
| 73 |
| 44 |
| x |
| 4.4g |
ϡ���������ʵ�����������
| 7.3g |
| 100g |
��ϡ���������ʵ���������Ϊ7.3%
�ʴ�Ϊ����1������©��������ƿ����2��A�� 2KMn04
| ||
��������������׳����ĵط����û�ѧ����֤��C02��NaOH��Һȷʵ�����˻�ѧ��Ӧ��Ҫ���ݶ�����̼���������Ʒ�Ӧ����̼���ƺ�ˮ�����ټ����Ƿ����̼������ӣ�����ͨ����̼������Ӳ���������Ҳ������̼������Ӳ�������Ҳ�ɣ�

��ϰ��ϵ�д�
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
�����Ŀ