��Ŀ����
��2009?������һģ���ڵ������������Ҫ�Ĵ����������ɻ�ѧ��Ӧ�����ģ���1�������������ڿ�������Դ���ǣ���д��ĸ��ţ�
A���Ҵ� B��ʯ�� C��ú D����Ȼ��
��2���Ҵ��׳ƾƾ�����ѧʽ��C2H5OH��������Է��������ǣ�23g�Ҵ��к���̼Ԫ��g��
��3��ʵ����ʹ���Ҵ���ΪҺ��ȼ�ϣ�ȼ�յĻ�ѧ����ʽ�ǣ�C2H5OH+3O2
 3H2O+2CO2��100g���Ҵ�92%�ľƾ��У����Ҵ���������g�����ȼ�տ�����CO2��������g��
3H2O+2CO2��100g���Ҵ�92%�ľƾ��У����Ҵ���������g�����ȼ�տ�����CO2��������g��
���𰸡���������1�������Ҵ���ʯ�͡�ú����Ȼ�����γɻ���ȡ�����ش�
��2�������Ҵ��Ļ�ѧʽ��C2H5OH���㣮
��3�������������������ļ��㹫ʽ����ǰһ�ʣ������Ҵ�ȼ�յĻ�ѧ����ʽ�����һ�ʣ�
����⣺��1��A���Ҵ������ס����������ӷ����Ƴɵģ��ǿ�������Դ��������ȷ��
B��ʯ���ǹŴ�������������徭�����긴�ӵı仯�γɵģ��ǻ�ʯ��Դ���������������Դ���
C��ú�ǹŴ�ֲ������徭�����긴�ӵı仯�γɵģ��ǻ�ʯ��Դ���������������Դ���
D����Ȼ���ǹŴ�������������徭�����긴�ӵı仯�γɵģ��ǻ�ʯ��Դ���������������Դ���
��2��C2H5OH����Է�������=12×2+1×5+16+1=46��23g�Ҵ��к���̼Ԫ�ص�����=23g×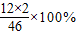 =12��
=12��
��3��100g���Ҵ�92%�ľƾ��У����Ҵ�������=100g×92%=92g��
�������CO2��������X
C2H5OH+3O2 3H2O+2CO2
3H2O+2CO2
46 88
92g X
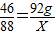
X=176g��
�ʴ�Ϊ����1��A��
��2��46��12��
��3��92��176��
��������Դ����Ϣ�����ϡ������ǵ��������Ĵ���Ҫ���⣬����ԴΣ���������صĽ��죬����Դ�Ŀ��������ã�����������ȵ㣬Ҳ�ǻ�ѧ������ȵ㣬�ر������ܡ��Ҵ����͵ȣ�
��2�������Ҵ��Ļ�ѧʽ��C2H5OH���㣮
��3�������������������ļ��㹫ʽ����ǰһ�ʣ������Ҵ�ȼ�յĻ�ѧ����ʽ�����һ�ʣ�
����⣺��1��A���Ҵ������ס����������ӷ����Ƴɵģ��ǿ�������Դ��������ȷ��
B��ʯ���ǹŴ�������������徭�����긴�ӵı仯�γɵģ��ǻ�ʯ��Դ���������������Դ���
C��ú�ǹŴ�ֲ������徭�����긴�ӵı仯�γɵģ��ǻ�ʯ��Դ���������������Դ���
D����Ȼ���ǹŴ�������������徭�����긴�ӵı仯�γɵģ��ǻ�ʯ��Դ���������������Դ���
��2��C2H5OH����Է�������=12×2+1×5+16+1=46��23g�Ҵ��к���̼Ԫ�ص�����=23g×
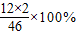 =12��
=12����3��100g���Ҵ�92%�ľƾ��У����Ҵ�������=100g×92%=92g��
�������CO2��������X
C2H5OH+3O2
 3H2O+2CO2
3H2O+2CO246 88
92g X
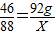
X=176g��
�ʴ�Ϊ����1��A��
��2��46��12��
��3��92��176��
��������Դ����Ϣ�����ϡ������ǵ��������Ĵ���Ҫ���⣬����ԴΣ���������صĽ��죬����Դ�Ŀ��������ã�����������ȵ㣬Ҳ�ǻ�ѧ������ȵ㣬�ر������ܡ��Ҵ����͵ȣ�

��ϰ��ϵ�д�
�����Ŀ
 ��2009?������һģ���û�ѧ�����ʾ�������ʻ�Ӧ��
��2009?������һģ���û�ѧ�����ʾ�������ʻ�Ӧ��