��Ŀ����
27����1��ijС�մ���Һ�к�16.8gNaHCO3������һ�����ĵ��ʻ���X��ǡ��ʹ��Һ������ֻ��Na2CO3��������д��X�Ļ�ѧʽ��������
���磺XΪ
��XΪ
��XΪ
��XΪ
��2����������Ϊ18.4g��NaOH��NaHCO3�Ļ���װ��һ�ܱ������У���120����¶��½��м��ȣ�����ַ�Ӧ���ų�ʣ�����壬��ʱ�����ڹ������ʵ�����Ϊ16.6g���Լ���ԭ�������NaOH��NaHCO3��������Ϊ���٣�
����ʾ��NaHCO3+NaOH120��Na2CO3+H2O��
���磺XΪ
NaOH
ʱ������Ϊ8g
����XΪ
��
ʱ������Ϊ4.6��
����XΪ
Na2O��
ʱ������Ϊ6.2��
����XΪ
Na2O2
ʱ������Ϊ7.8��
����2����������Ϊ18.4g��NaOH��NaHCO3�Ļ���װ��һ�ܱ������У���120����¶��½��м��ȣ�����ַ�Ӧ���ų�ʣ�����壬��ʱ�����ڹ������ʵ�����Ϊ16.6g���Լ���ԭ�������NaOH��NaHCO3��������Ϊ���٣�
����ʾ��NaHCO3+NaOH120��Na2CO3+H2O��
���������ȷ�����Ӧԭ������Ϊʵ���ܱ������н��з�Ӧ�����Դ��ڹ������⣬��Ϊ���߿��Է�����ӦNaOH+NaHCO3 $\frac{\underline{\;����\;}}{\;}$Na2CO3+H2O����Ӧ�������������һ��ǡ�÷�Ӧ��ȫ�������������ƹ��������������Ӧ������̼�����ƹ�����ʣ���̼�����ƻ�Ҫ�����ֽⷴӦ��
���Խ���������Ҫͨ��ǡ�÷�Ӧ���ж��Ƿ������ȷ����Ӧ����Ȼ���ٸ��ݻ�ѧ����ʽ�������������ʵ�����ȥ���н��
���Խ���������Ҫͨ��ǡ�÷�Ӧ���ж��Ƿ������ȷ����Ӧ����Ȼ���ٸ��ݻ�ѧ����ʽ�������������ʵ�����ȥ���н��
����⣺��1��ijС�մ���Һ�к�16.8gNaHCO3������һ�����ĵ��ʻ���X��ǡ��ʹ��Һ������ֻ��Na2CO3��������д��X�Ļ�ѧʽ��������
���磺XΪ NaOHʱ������Ϊ 8g��
��XΪ ��ʱ������Ϊ 4.6�ˣ�
��XΪ Na2O��ʱ������Ϊ 6.2�ˣ�
��XΪ Na2O2ʱ������Ϊ 7.8�ˣ�
��2��������֪��Ӧǰ�����������ͷ�Ӧ���������������ԣ��п��ܴ��ڹ������⣮
��18.4g�����������ȫ��Ӧ�����ٵ�����Ϊx��
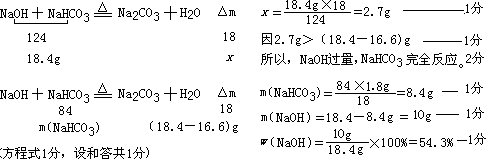
��ԭ�������NaOH����������Ϊ54.3%��
�ʴ�Ϊ����1����XΪ ��ʱ������Ϊ 4.6�ˣ�
��XΪ Na2O��ʱ������Ϊ 6.2�ˣ�
��XΪ Na2O2ʱ������Ϊ 7.8�ˣ�
��2��ԭ�������NaOH��NaHCO3��������Ϊ10�˺�8.4�ˣ�
���磺XΪ NaOHʱ������Ϊ 8g��
��XΪ ��ʱ������Ϊ 4.6�ˣ�
��XΪ Na2O��ʱ������Ϊ 6.2�ˣ�
��XΪ Na2O2ʱ������Ϊ 7.8�ˣ�
��2��������֪��Ӧǰ�����������ͷ�Ӧ���������������ԣ��п��ܴ��ڹ������⣮
��18.4g�����������ȫ��Ӧ�����ٵ�����Ϊx��
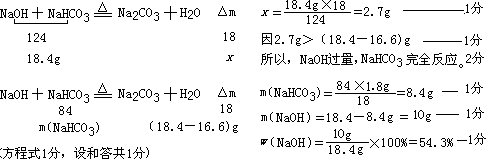
��ԭ�������NaOH����������Ϊ54.3%��
�ʴ�Ϊ����1����XΪ ��ʱ������Ϊ 4.6�ˣ�
��XΪ Na2O��ʱ������Ϊ 6.2�ˣ�
��XΪ Na2O2ʱ������Ϊ 7.8�ˣ�
��2��ԭ�������NaOH��NaHCO3��������Ϊ10�˺�8.4�ˣ�
�����������ѶȺܴ�һ���жϷ�Ӧ�������⣬���������жϹ��������жϹ�����Ϊʲô2.7g����18.4g-16.6g�������ж�Ϊ�������ƹ����أ���Ϊ����̼�����ƹ������ͻ�����ֽ⣬���������������٣������ⲻ����

��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
�����Ŀ